当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroxylation, Epoxidation, and Dehydrogenation of Capsaicin by a Microbial Promiscuous Cytochrome P450 105D7
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-03-03 , DOI: 10.1002/cbdv.202000910 Bingbing Ma 1 , Qianwen Wang 1 , Bing-Nan Han 2 , Haruo Ikeda 3 , Chunfang Zhang 1 , Lian-Hua Xu 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-03-03 , DOI: 10.1002/cbdv.202000910 Bingbing Ma 1 , Qianwen Wang 1 , Bing-Nan Han 2 , Haruo Ikeda 3 , Chunfang Zhang 1 , Lian-Hua Xu 2
Affiliation
|
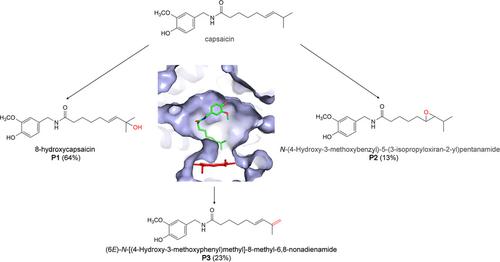
Cytochrome P450 enzymes (P450s) are versatile biocatalysts, which insert a molecular oxygen into inactivated C−H bonds under mild conditions. CYP105D7 from Streptomyces avermitilis has been reported as a bacterial substrate‐promiscuous P450 which catalyzes the hydroxylation of 1‐deoxypentalenic acid, diclofenac, naringenin, compactin and steroids. In this study, CYP105D7 catalyzes hydroxylation, epoxidation and dehydrogenation of capsaicin, a pharmaceutical agent, revealing its functional diversity. The kinetic parameters of the CYP105D7 oxidation of capsaicin were determined as Km=311.60±87.30 μM and kcat=2.01±0.33 min−1. In addition, we conducted molecular docking, mutagenesis and substrate binding analysis, indicating that Arg81 plays crucial role in the capsaicin binding and catalysis. To our best knowledge, this study presents the first report to illustrate that capsaicin can be catalyzed by prokaryotic P450s.
中文翻译:

微生物混杂细胞色素P450 105D7对辣椒素的羟化,环氧化和脱氢作用
细胞色素P450酶(P450s)是通用的生物催化剂,可在温和条件下将分子氧插入失活的CH键中。据报道,来自阿维链霉菌的CYP105D7是一种细菌底物-混杂P450,可催化1-脱氧戊酸,双氯芬酸,柚皮素,致密蛋白和类固醇的羟化反应。在这项研究中,CYP105D7催化辣椒素(一种药物)的羟化,环氧化和脱氢反应,从而揭示其功能多样性。确定辣椒素的CYP105D7氧化动力学参数为K m = 311.60±87.30μM和k cat = 2.01±0.33 min -1。此外,我们进行了分子对接,诱变和底物结合分析,表明Arg81在辣椒素结合和催化中起着至关重要的作用。据我们所知,本研究提出了第一个报告,以说明辣椒素可以被原核P450催化。
更新日期:2021-04-14
中文翻译:

微生物混杂细胞色素P450 105D7对辣椒素的羟化,环氧化和脱氢作用
细胞色素P450酶(P450s)是通用的生物催化剂,可在温和条件下将分子氧插入失活的CH键中。据报道,来自阿维链霉菌的CYP105D7是一种细菌底物-混杂P450,可催化1-脱氧戊酸,双氯芬酸,柚皮素,致密蛋白和类固醇的羟化反应。在这项研究中,CYP105D7催化辣椒素(一种药物)的羟化,环氧化和脱氢反应,从而揭示其功能多样性。确定辣椒素的CYP105D7氧化动力学参数为K m = 311.60±87.30μM和k cat = 2.01±0.33 min -1。此外,我们进行了分子对接,诱变和底物结合分析,表明Arg81在辣椒素结合和催化中起着至关重要的作用。据我们所知,本研究提出了第一个报告,以说明辣椒素可以被原核P450催化。































 京公网安备 11010802027423号
京公网安备 11010802027423号