当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CRISPR technology incorporating amplification strategies: molecular assays for nucleic acids, proteins, and small molecules
Chemical Science ( IF 7.6 ) Pub Date : 2021-3-2 , DOI: 10.1039/d0sc06973f Wei Feng 1 , Ashley M Newbigging 1 , Jeffrey Tao 1 , Yiren Cao 1 , Hanyong Peng 1 , Connie Le 2 , Jinjun Wu 1 , Bo Pang 1, 3 , Juan Li 3 , D Lorne Tyrrell 2 , Hongquan Zhang 1 , X Chris Le 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-3-2 , DOI: 10.1039/d0sc06973f Wei Feng 1 , Ashley M Newbigging 1 , Jeffrey Tao 1 , Yiren Cao 1 , Hanyong Peng 1 , Connie Le 2 , Jinjun Wu 1 , Bo Pang 1, 3 , Juan Li 3 , D Lorne Tyrrell 2 , Hongquan Zhang 1 , X Chris Le 1
Affiliation
|
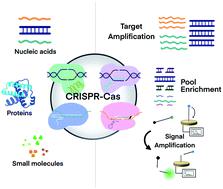
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) protein systems have transformed the field of genome editing and transcriptional modulation. Progress in CRISPR–Cas technology has also advanced molecular detection of diverse targets, ranging from nucleic acids to proteins. Incorporating CRISPR–Cas systems with various nucleic acid amplification strategies enables the generation of amplified detection signals, enrichment of low-abundance molecular targets, improvements in analytical specificity and sensitivity, and development of point-of-care (POC) diagnostic techniques. These systems take advantage of various Cas proteins for their particular features, including RNA-guided endonuclease activity, sequence-specific recognition, multiple turnover trans-cleavage activity of Cas12 and Cas13, and unwinding and nicking ability of Cas9. Integrating a CRISPR–Cas system after nucleic acid amplification improves detection specificity due to RNA-guided recognition of specific sequences of amplicons. Incorporating CRISPR–Cas before nucleic acid amplification enables enrichment of rare and low-abundance nucleic acid targets and depletion of unwanted abundant nucleic acids. Unwinding of dsDNA to ssDNA using CRISPR–Cas9 at a moderate temperature facilitates techniques for achieving isothermal exponential amplification of nucleic acids. A combination of CRISPR–Cas systems with functional nucleic acids (FNAs) and molecular translators enables the detection of non-nucleic acid targets, such as proteins, metal ions, and small molecules. Successful integrations of CRISPR technology with nucleic acid amplification techniques result in highly sensitive and rapid detection of SARS-CoV-2, the virus that causes the COVID-19 pandemic.
中文翻译:

结合扩增策略的 CRISPR 技术:核酸、蛋白质和小分子的分子测定
成簇规则间隔短回文重复序列 (CRISPR) 和 CRISPR 相关 (Cas) 蛋白质系统已经改变了基因组编辑和转录调节领域。CRISPR-Cas 技术的进步也促进了从核酸到蛋白质等多种靶标的分子检测。将 CRISPR-Cas 系统与各种核酸扩增策略相结合,可以生成放大的检测信号、富集低丰度分子靶标、提高分析特异性和灵敏度以及开发即时 (POC) 诊断技术。这些系统利用各种 Cas 蛋白的特殊功能,包括 RNA 引导的核酸内切酶活性、序列特异性识别、Cas12 和 Cas13 的多次翻转反式切割活性以及 Cas9 的解旋和切口能力。由于 RNA 引导识别特定的扩增子序列,核酸扩增后整合 CRISPR-Cas 系统可提高检测特异性。在核酸扩增之前加入 CRISPR-Cas 可以富集稀有和低丰度的核酸靶标,并消除不需要的丰富核酸。在中等温度下使用 CRISPR-Cas9 将 dsDNA 解旋为 ssDNA 有助于实现核酸等温指数扩增的技术。CRISPR-Cas 系统与功能性核酸 (FNA) 和分子翻译器的组合能够检测非核酸靶标,例如蛋白质、金属离子和小分子。CRISPR 技术与核酸扩增技术的成功整合可实现对 SARS-CoV-2(导致 COVID-19 大流行的病毒)的高度灵敏和快速检测。
更新日期:2021-03-02
中文翻译:

结合扩增策略的 CRISPR 技术:核酸、蛋白质和小分子的分子测定
成簇规则间隔短回文重复序列 (CRISPR) 和 CRISPR 相关 (Cas) 蛋白质系统已经改变了基因组编辑和转录调节领域。CRISPR-Cas 技术的进步也促进了从核酸到蛋白质等多种靶标的分子检测。将 CRISPR-Cas 系统与各种核酸扩增策略相结合,可以生成放大的检测信号、富集低丰度分子靶标、提高分析特异性和灵敏度以及开发即时 (POC) 诊断技术。这些系统利用各种 Cas 蛋白的特殊功能,包括 RNA 引导的核酸内切酶活性、序列特异性识别、Cas12 和 Cas13 的多次翻转反式切割活性以及 Cas9 的解旋和切口能力。由于 RNA 引导识别特定的扩增子序列,核酸扩增后整合 CRISPR-Cas 系统可提高检测特异性。在核酸扩增之前加入 CRISPR-Cas 可以富集稀有和低丰度的核酸靶标,并消除不需要的丰富核酸。在中等温度下使用 CRISPR-Cas9 将 dsDNA 解旋为 ssDNA 有助于实现核酸等温指数扩增的技术。CRISPR-Cas 系统与功能性核酸 (FNA) 和分子翻译器的组合能够检测非核酸靶标,例如蛋白质、金属离子和小分子。CRISPR 技术与核酸扩增技术的成功整合可实现对 SARS-CoV-2(导致 COVID-19 大流行的病毒)的高度灵敏和快速检测。

































 京公网安备 11010802027423号
京公网安备 11010802027423号