当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Useful Synthetic Route to N-Nonsubstituted Succinimides via Light-Induced Degradation of Metallocarbonyl Complexes
Organometallics ( IF 2.5 ) Pub Date : 2021-03-01 , DOI: 10.1021/acs.organomet.0c00768 Aneta Kosińska 1 , Sławomir Wojtulewski 2 , Marcin Palusiak 3 , Paweł Tokarz 4 , Bogna Rudolf 1
Organometallics ( IF 2.5 ) Pub Date : 2021-03-01 , DOI: 10.1021/acs.organomet.0c00768 Aneta Kosińska 1 , Sławomir Wojtulewski 2 , Marcin Palusiak 3 , Paweł Tokarz 4 , Bogna Rudolf 1
Affiliation
|
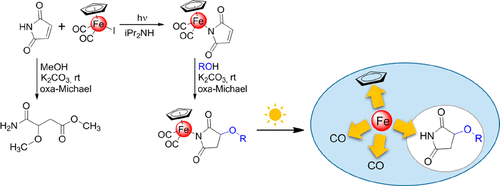
Succinimides are among the most studied compounds due to their wide profile of biological activities. It is well-known that succinimides undergo ring-opening reactions under alkaline conditions. This feature limits the formation of 3-substituted succinimides from maleimides by an oxa-Michael reaction, for which basic conditions are required. Herein, we report the synthesis, characterization, and crystal structure of metallocarbonyl (Fe, Ru) complexes bearing 3-substituted succinimide ligands. These complexes were obtained in oxa-Michael reactions of CpM(CO)2(η1-maleimidato) (M = Fe, Ru) with alcohols (MeOH, EtOH) in the presence of K2CO3. During the crystallographic studies of an iron complex bearing the 3-methoxysuccinimide ligand, we also identified unexpected crystals of free 3-methoxysuccinimide. We performed some additional experiments and theoretical calculations to determine the mechanism of formation of the obtained N-nonsubstituted succinimide. We demonstrate that 3-methoxysuccinimide resulted from light-induced degradation of CpFe(CO)2(η1-3-methoxysuccinimidato). On the basis of these findings, we propose an indirect route leading to 3-substituted succinimides starting from maleimide via the light-induced degradation of iron metallocarbonyl succinimidato complexes. The proposed methodology prevents ring opening of succinimide under alkaline conditions and gives N-nonsubstituted succinimide products. To confirm the effectiveness of the described method, CpFe(CO)2(η1-maleimidato) was allowed to react with several aliphatic alcohols and phenol, affording the oxa-Michael reaction products in the case of primary alcohols. The irradiation of the obtained compounds with daylight gave the N-nonsubstituted 3-alkoxysuccinimides.
中文翻译:

通过光诱导的金属羰基配合物降解制备N-非取代的琥珀酰亚胺的有用合成路线
琥珀酰亚胺由于其广泛的生物活性而成为研究最多的化合物之一。众所周知,琥珀酰亚胺在碱性条件下会发生开环反应。该特征限制了通过oxa-Michael反应由马来酰亚胺形成3-取代的琥珀酰亚胺的条件,这是基本条件。本文中,我们报道了带有3个取代的琥珀酰亚胺配体的金属羰基(Fe,Ru)配合物的合成,表征和晶体结构。在CPM的氧杂迈克尔反应,获得这些络合物(CO)2(η 1 -maleimidato)(M =铁,钌)与醇类(甲醇,乙醇)中K的存在2 CO 3。在带有3-甲氧基琥珀酰亚胺配体的铁配合物的晶体学研究中,我们还发现了游离的3-甲氧基琥珀酰亚胺的出乎意料的晶体。我们进行了一些额外的实验和理论计算,以确定所获得的N-非取代的琥珀酰亚胺的形成机理。我们证明了3-methoxysuccinimide源于(CO)CpFe的量的光诱导降解2(η 1 -3- methoxysuccinimidato)。基于这些发现,我们提出了一种间接途径,其导致马来酰亚胺开始的3-取代的琥珀酰亚胺通过光诱导的金属羰基琥珀酰亚胺铁络合物的降解而产生。所提出的方法可防止碱性条件下琥珀酰亚胺的开环并产生N-非取代的琥珀酰亚胺产品。为了证实所描述的方法的有效性,CpFe的量(CO)2(η 1 -maleimidato)被允许与几个脂族醇和酚反应,得到的氧杂迈克尔反应产物在伯醇的情况下。用日光照射获得的化合物,得到N-未取代的3-烷氧基琥珀酰亚胺。
更新日期:2021-03-22
中文翻译:

通过光诱导的金属羰基配合物降解制备N-非取代的琥珀酰亚胺的有用合成路线
琥珀酰亚胺由于其广泛的生物活性而成为研究最多的化合物之一。众所周知,琥珀酰亚胺在碱性条件下会发生开环反应。该特征限制了通过oxa-Michael反应由马来酰亚胺形成3-取代的琥珀酰亚胺的条件,这是基本条件。本文中,我们报道了带有3个取代的琥珀酰亚胺配体的金属羰基(Fe,Ru)配合物的合成,表征和晶体结构。在CPM的氧杂迈克尔反应,获得这些络合物(CO)2(η 1 -maleimidato)(M =铁,钌)与醇类(甲醇,乙醇)中K的存在2 CO 3。在带有3-甲氧基琥珀酰亚胺配体的铁配合物的晶体学研究中,我们还发现了游离的3-甲氧基琥珀酰亚胺的出乎意料的晶体。我们进行了一些额外的实验和理论计算,以确定所获得的N-非取代的琥珀酰亚胺的形成机理。我们证明了3-methoxysuccinimide源于(CO)CpFe的量的光诱导降解2(η 1 -3- methoxysuccinimidato)。基于这些发现,我们提出了一种间接途径,其导致马来酰亚胺开始的3-取代的琥珀酰亚胺通过光诱导的金属羰基琥珀酰亚胺铁络合物的降解而产生。所提出的方法可防止碱性条件下琥珀酰亚胺的开环并产生N-非取代的琥珀酰亚胺产品。为了证实所描述的方法的有效性,CpFe的量(CO)2(η 1 -maleimidato)被允许与几个脂族醇和酚反应,得到的氧杂迈克尔反应产物在伯醇的情况下。用日光照射获得的化合物,得到N-未取代的3-烷氧基琥珀酰亚胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号