当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-Phase Quasi-Intramolecular Redox Reaction of [Ag(NH3)2]MnO4: An Easy Way to Prepare Pure AgMnO2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-01 , DOI: 10.1021/acs.inorgchem.0c03498
Lara A Fogaca 1, 2 , Éva Kováts 3 , Gergely Németh 3 , Katalin Kamarás 3 , Kende A Béres 2 , Péter Németh 2, 4 , Vladimir Petruševski 5 , Laura Bereczki 6 , Berta Barta Holló 7 , István E Sajó 8 , Szilvia Klébert 2 , Attila Farkas 9 , Imre M Szilágyi 1 , László Kótai 2, 10
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-01 , DOI: 10.1021/acs.inorgchem.0c03498
Lara A Fogaca 1, 2 , Éva Kováts 3 , Gergely Németh 3 , Katalin Kamarás 3 , Kende A Béres 2 , Péter Németh 2, 4 , Vladimir Petruševski 5 , Laura Bereczki 6 , Berta Barta Holló 7 , István E Sajó 8 , Szilvia Klébert 2 , Attila Farkas 9 , Imre M Szilágyi 1 , László Kótai 2, 10
Affiliation
|
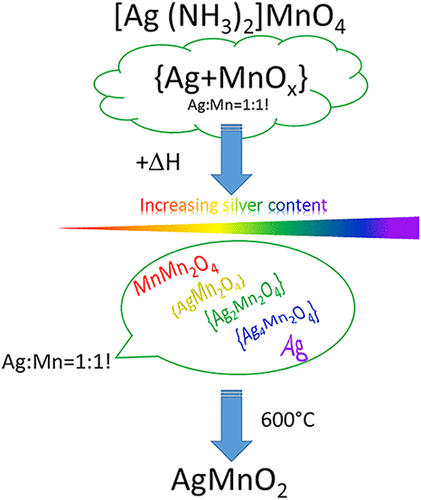
Two monoclinic polymorphs of [Ag(NH3)2]MnO4 containing a unique coordination mode of permanganate ions were prepared, and the high-temperature polymorph was used as a precursor to synthesize pure AgMnO2. The hydrogen bonds between the permanganate ions and the hydrogen atoms of ammonia were detected by IR spectroscopy and single-crystal X-ray diffraction. Under thermal decomposition, these hydrogen bonds induced a solid-phase quasi-intramolecular redox reaction between the [Ag(NH3)2]+ cation and MnO4– anion even before losing the ammonia ligand or permanganate oxygen atom. The polymorphs decomposed into finely dispersed elementary silver, amorphous MnOx compounds, and H2O, N2 and NO gases. Annealing the primary decomposition product at 573 K, the metallic silver reacted with the manganese oxides and resulted in the formation of amorphous silver manganese oxides, which started to crystallize only at 773 K and completely transformed into AgMnO2 at 873 K.
中文翻译:

[Ag(NH3)2]MnO4 的固相准分子内氧化还原反应:制备纯 AgMnO2 的简单方法
制备了两种含有独特高锰酸根离子配位模式的单斜晶型[Ag(NH 3 ) 2 ]MnO 4 ,并以高温多晶型为前驱体合成了纯AgMnO 2 。通过红外光谱和单晶X射线衍射检测了高锰酸根离子与氨的氢原子之间的氢键。在热分解下,这些氢键甚至在失去氨配体或高锰酸盐氧原子之前就诱导了[Ag(NH 3 ) 2 ] +阳离子和MnO 4 -阴离子之间的固相准分子内氧化还原反应。多晶型物分解成细分散的单质银、无定形MnO x化合物以及H 2 O、N 2和NO气体。在 573 K 下对初级分解产物进行退火,金属银与锰氧化物反应,形成非晶态银锰氧化物,仅在 773 K 时开始结晶,并在 873 K 时完全转化为 AgMnO 2 。
更新日期:2021-03-15
中文翻译:

[Ag(NH3)2]MnO4 的固相准分子内氧化还原反应:制备纯 AgMnO2 的简单方法
制备了两种含有独特高锰酸根离子配位模式的单斜晶型[Ag(NH 3 ) 2 ]MnO 4 ,并以高温多晶型为前驱体合成了纯AgMnO 2 。通过红外光谱和单晶X射线衍射检测了高锰酸根离子与氨的氢原子之间的氢键。在热分解下,这些氢键甚至在失去氨配体或高锰酸盐氧原子之前就诱导了[Ag(NH 3 ) 2 ] +阳离子和MnO 4 -阴离子之间的固相准分子内氧化还原反应。多晶型物分解成细分散的单质银、无定形MnO x化合物以及H 2 O、N 2和NO气体。在 573 K 下对初级分解产物进行退火,金属银与锰氧化物反应,形成非晶态银锰氧化物,仅在 773 K 时开始结晶,并在 873 K 时完全转化为 AgMnO 2 。































 京公网安备 11010802027423号
京公网安备 11010802027423号