当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface vs Homogeneous Organo-Hafnium Catalyst Ion-Pairing and Ligand Effects on Ethylene Homo- and Copolymerizations
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-26 , DOI: 10.1021/acscatal.0c04678 Jialong Zhang 1 , Alexander H. Mason 1 , Alessandro Motta 2 , Laryssa G. Cesar 3 , Yosi Kratish 1 , Tracy L. Lohr 1 , Jeffrey T. Miller 3 , Yanshan Gao 1 , Tobin J. Marks 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-26 , DOI: 10.1021/acscatal.0c04678 Jialong Zhang 1 , Alexander H. Mason 1 , Alessandro Motta 2 , Laryssa G. Cesar 3 , Yosi Kratish 1 , Tracy L. Lohr 1 , Jeffrey T. Miller 3 , Yanshan Gao 1 , Tobin J. Marks 1
Affiliation
|
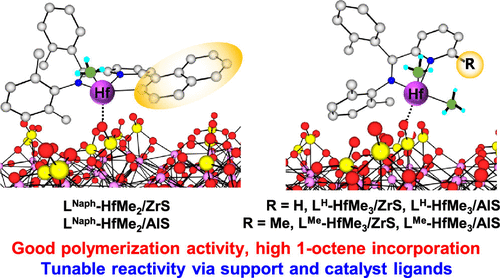
Heterogeneous catalysts have long dominated polyethylene and polypropylene production, but understanding their catalysis is challenged by uncertainties in active site structures and percentages. Surface-bound organometallic catalysts are an emerging strategy to combine successful homogeneous catalysts having well-understood structures, relatively high percentages of active sites, and exceptional control of selectivity, with the attractions of surface catalysts, to transition promising homogeneous systems to large-scale heterogeneous ones. Nevertheless, surface-bound olefin polymerization catalysts typically produce ultrahigh Mw’s but with low activity and comonomer selectivity. Here, we report the systematic synthesis and characterization of a series of pyridylamido–Hf complexes and their corresponding surface catalysts chemisorbed on sulfated alumina (AlS) and zirconia (ZrS). Comparative ethylene homo- and 1-octene copolymerizations reveal similar activity and 1-octene selectivity trends in the homogeneous and heterogeneous systems. For the surface pyridylamido–Hf catalyst series, large variations in activity (up to 10×) and 1-octene incorporation (up to 28×) are achieved by ligand and support manipulation. Interestingly, while the homogeneous catalysts exhibit positive comonomer effects in ethylene/1-octene copolymerization, the surface catalysts behave oppositely. Extended X-ray absorption fine structure (EXAFS) reveals significantly elongated Hf···O bond distances vs typical Hf–O covalent bonds (2.06 vs 1.97 Å). Density functional theory (DFT) analysis of the heterolytic ion pair separation enthalpies, olefin insertion energetics, and NBO/Bader charges also suggest electrostatic Hf cation–anionic support binding and catalytic patterns, which are modulated by the ion-pairing energetics and ligand architecture.
中文翻译:

表面与均相有机-催化剂的离子配对和配体对乙烯均聚和共聚的影响
多相催化剂长期以来一直主导着聚乙烯和聚丙烯的生产,但了解其催化作用受到活性位点结构和百分比不确定性的挑战。表面结合的有机金属催化剂是一种新兴的策略,可以将具有良好理解的结构,相对较高的活性位点百分比和选择性的优异控制的成功的均相催化剂与表面催化剂相结合,以将有前途的均相系统转变为大规模的均相系统那些。然而,表面结合的烯烃聚合催化剂通常会产生超高的M w具有低活性和共聚单体选择性。在这里,我们报告了一系列吡啶基-Hf络合物及其化学吸附在硫酸化氧化铝(AlS)和氧化锆(ZrS)上的相应表面催化剂的系统合成和表征。比较的乙烯均聚和1-辛烯共聚显示出均相和非均相体系中相似的活性和1-辛烯的选择性趋势。对于表面吡啶基-Hf催化剂系列,配体和载体操作可实现活性(最多10倍)和1-辛烯掺入(最多28倍)的大变化。有趣的是,尽管均相催化剂在乙烯/ 1-辛烯共聚中显示出积极的共聚单体效果,但表面催化剂的行为却相反。扩展的X射线吸收精细结构(EXAFS)显示,与典型的Hf-O共价键(2.06对1.97Å)相比,Hf··O的键距明显延长。对杂合离子对分离焓,烯烃插入能和NBO / Bader电荷的密度泛函理论(DFT)分析还表明,静电Hf阳离子-阴离子载体的结合和催化模式受离子对能和配体结构的调节。
更新日期:2021-03-19
中文翻译:

表面与均相有机-催化剂的离子配对和配体对乙烯均聚和共聚的影响
多相催化剂长期以来一直主导着聚乙烯和聚丙烯的生产,但了解其催化作用受到活性位点结构和百分比不确定性的挑战。表面结合的有机金属催化剂是一种新兴的策略,可以将具有良好理解的结构,相对较高的活性位点百分比和选择性的优异控制的成功的均相催化剂与表面催化剂相结合,以将有前途的均相系统转变为大规模的均相系统那些。然而,表面结合的烯烃聚合催化剂通常会产生超高的M w具有低活性和共聚单体选择性。在这里,我们报告了一系列吡啶基-Hf络合物及其化学吸附在硫酸化氧化铝(AlS)和氧化锆(ZrS)上的相应表面催化剂的系统合成和表征。比较的乙烯均聚和1-辛烯共聚显示出均相和非均相体系中相似的活性和1-辛烯的选择性趋势。对于表面吡啶基-Hf催化剂系列,配体和载体操作可实现活性(最多10倍)和1-辛烯掺入(最多28倍)的大变化。有趣的是,尽管均相催化剂在乙烯/ 1-辛烯共聚中显示出积极的共聚单体效果,但表面催化剂的行为却相反。扩展的X射线吸收精细结构(EXAFS)显示,与典型的Hf-O共价键(2.06对1.97Å)相比,Hf··O的键距明显延长。对杂合离子对分离焓,烯烃插入能和NBO / Bader电荷的密度泛函理论(DFT)分析还表明,静电Hf阳离子-阴离子载体的结合和催化模式受离子对能和配体结构的调节。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号