Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-12-31 , DOI: 10.2174/1570180817999200819122350
Monika Rakse 1 , Chandrabose Karthikeyan 2 , Narayana Subbiah Hari Narayana Moorthy 2 , Ram Kishore Agrawal 1
|
Background: Protein Tyrosine Phosphatase 1B (PTP1B) is an attractive target for antidiabetic drug discovery owing to its pivotal role as a negative regulator of insulin and leptin signaling.
Objective: The objective of this research is to design, synthesize, and evaluate some acetamidobenzoic acid derivatives as a novel class of protein tyrosine phosphatase 1B inhibitors with therapeutic potential for Type II diabetes.
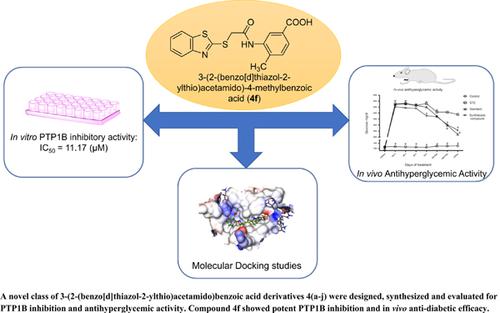
Methods: 3-(2-(Benzo[d]thiazol-2-ylthio)acetamido)benzoic acid derivatives 4(a-j) were synthesized and characterized by employing spectral studies. All the synthesized compounds were screened for in vitro PTP1B inhibitory activity and the most potent compound in the series was also evaluated for in vivo anti-hyperglycemic activity using STZ induced diabetic Wistar rat model. Molecular docking studies were also performed with the most potent analog using FlexX docking algorithm to delineate its binding mode to the active site of the PTP1B.
Results: Among all the synthesized compounds, 3-(2-(benzo[d]thiazol-2-ylthio)acetamido)-4- methylbenzoic acid (4f) displayed good PTP1B inhibitory activity with an IC50 value of 11.17 μM. The compound also exhibited good anti hyperglycemic efficacy in streptozotocin induced diabetic Wistar rats. Docking studies with 4f revealed that the compound bound in the catalytic and second aryl binding site of the PTP1B.
Conclusion: Overall, compound 4f with good in vitro PTP1B inhibitory potency and in vivo antihyperglycemic efficacy would be a valuable lead molecule for the development of acetamidobenzoic acid based PTP1B inhibitors with antidiabetic potential.
中文翻译:

3-(2-(苯并[d]噻唑-2-基硫基)乙酰氨基)苯甲酸衍生物作为蛋白酪氨酸磷酸酶1B抑制剂的设计,合成及生物学评价
背景:酪氨酸磷酸酶1B(PTP1B)由于其作为胰岛素和瘦素信号传导的负调节剂的关键作用,是抗糖尿病药物发现的有吸引力的靶标。
目的:本研究的目的是设计,合成和评估一些乙酰氨基苯甲酸衍生物,它们是一类新型的蛋白酪氨酸磷酸酶1B抑制剂,具有治疗II型糖尿病的潜力。
方法:合成3-(2-(苯并[d]噻唑-2-基硫基)乙酰氨基)苯甲酸衍生物4(aj),并通过光谱研究对其进行表征。筛选所有合成的化合物的体外PTP1B抑制活性,并使用STZ诱导的糖尿病Wistar大鼠模型评价该系列中最有效的化合物的体内抗高血糖活性。还使用FlexX对接算法对最有效的类似物进行了分子对接研究,以描述其与PTP1B活性位点的结合模式。
结果:在所有合成的化合物中,3-(2-(苯并[d]噻唑-2-基硫基)乙酰胺基)-4-甲基苯甲酸(4f)显示出良好的PTP1B抑制活性,IC50值为11.17μM。该化合物在链脲佐菌素诱导的糖尿病Wistar大鼠中也表现出良好的抗高血糖功效。与4f的对接研究表明,该化合物结合在PTP1B的催化和第二个芳基结合位点上。
结论:总的来说,具有良好的体外PTP1B抑制能力和体内抗高血糖功效的化合物4f将是开发基于乙酰氨基苯甲酸的具有抗糖尿病潜力的PTP1B抑制剂的有价值的先导分子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号