当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed Evolution of l-Threonine Aldolase for the Diastereoselective Synthesis of β-Hydroxy-α-amino Acids
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-25 , DOI: 10.1021/acscatal.0c04949 Wenlong Zheng 1, 2 , Haoran Yu 1, 2 , Sai Fang 1 , Kaitong Chen 1 , Zhe Wang 3 , Xiuli Cheng 3 , Gang Xu 1 , Lirong Yang 1, 2 , Jianping Wu 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-25 , DOI: 10.1021/acscatal.0c04949 Wenlong Zheng 1, 2 , Haoran Yu 1, 2 , Sai Fang 1 , Kaitong Chen 1 , Zhe Wang 3 , Xiuli Cheng 3 , Gang Xu 1 , Lirong Yang 1, 2 , Jianping Wu 1, 2
Affiliation
|
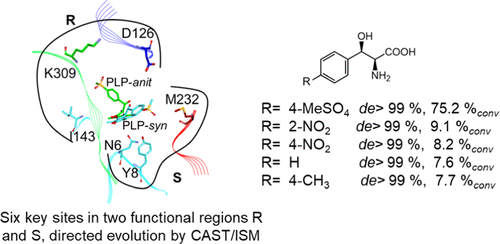
l-Threonine aldolase (LTA) is an attractive tool in organic chemistry for catalyzing the formation of β-hydroxy-α-amino acids with two chiral centers. The enzyme has a strict selectivity for Cα of β-hydroxy-α-amino acids but a moderate selectivity for Cβ, limiting its wide applications in stereospecific carbon–carbon bond synthesis. Here, a combinatorial active-site saturation test/iterative saturation mutagenesis (CAST/ISM) strategy was applied to accelerate directed evolution of LTA in diastereoselectivity. A total of 27 amino acid residues lining the substrate pocket were selected and divided into two groups based on their functional region. In silico screening and site-directed saturation mutagenesis identified six (3 + 3) amino acid residues of them with a significant effect on diastereoselectivity. The ISM strategy was then performed in and between each group to obtain the best combinatorial mutation. As a result, a variant RS1 (Y8H/Y31H/I143R/N305R) was obtained with a dramatically improved preference for the synthesis of l-syn-3-[4-(methylsulfonyl)phenylserine]. The product with a de value of 99.5% (73.2% conv) was produced in a 20 L reactor, which is promising in the industrial synthesis of aromatic l-syn-β-hydroxy-α-amino acids with LTA. The variant also represented a significant selective improvement to other l-phenylserine derivatives. The de values of 2-NO2-, 4-NO2-, H-, and 4-CH4-substituted l-phenylserine derivatives were more than 99%syn by dynamic control. The insight of the mutant model suggests that the binding pocket of the active center was reshaped.
中文翻译:

l-苏氨酸醛缩酶的定向进化,用于β-羟基-α-氨基酸的非对映选择性合成
1-苏氨酸醛缩酶(LTA)是有机化学中一种有吸引力的工具,用于催化具有两个手性中心的β-羟基-α-氨基酸的形成。酶具有对C严格的选择性α的β羟基α氨基酸,但对于C适度选择性β ,限制在立体有择的碳-碳键合成其广泛的应用。在这里,组合的主动站点饱和度测试/迭代饱和度诱变(CAST / ISM)策略用于加速非对映选择性中LTA的定向进化。共有27个氨基酸残基位于底物袋中,并根据其功能区域分为两组。在计算机上筛选和定点饱和诱变确定了其中的六个(3 + 3)氨基酸残基,对非对映选择性具有重大影响。然后在每个组中和组之间执行ISM策略以获得最佳组合突变。结果,获得了变体RS1(Y8H / Y31H / I143R / N305R),其对1 - syn -3- [4-(甲基磺酰基)苯基丝氨酸]的合成具有显着改善的偏好。在20 L反应器中生产出de值为99.5%(73.2%conv)的产品,这在用LTA工业合成芳族1 - syn -β-羟基-α-氨基酸方面很有希望。所述变体还表示的显著选择性改善其他升-苯基丝氨酸衍生物。通过动态控制,2-NO 2-,4-NO 2-,H-和4-CH 4-取代的1-苯基丝氨酸衍生物的de值大于99%syn。突变模型的见解表明,活性中心的结合口袋被重塑了。
更新日期:2021-03-19
中文翻译:

l-苏氨酸醛缩酶的定向进化,用于β-羟基-α-氨基酸的非对映选择性合成
1-苏氨酸醛缩酶(LTA)是有机化学中一种有吸引力的工具,用于催化具有两个手性中心的β-羟基-α-氨基酸的形成。酶具有对C严格的选择性α的β羟基α氨基酸,但对于C适度选择性β ,限制在立体有择的碳-碳键合成其广泛的应用。在这里,组合的主动站点饱和度测试/迭代饱和度诱变(CAST / ISM)策略用于加速非对映选择性中LTA的定向进化。共有27个氨基酸残基位于底物袋中,并根据其功能区域分为两组。在计算机上筛选和定点饱和诱变确定了其中的六个(3 + 3)氨基酸残基,对非对映选择性具有重大影响。然后在每个组中和组之间执行ISM策略以获得最佳组合突变。结果,获得了变体RS1(Y8H / Y31H / I143R / N305R),其对1 - syn -3- [4-(甲基磺酰基)苯基丝氨酸]的合成具有显着改善的偏好。在20 L反应器中生产出de值为99.5%(73.2%conv)的产品,这在用LTA工业合成芳族1 - syn -β-羟基-α-氨基酸方面很有希望。所述变体还表示的显著选择性改善其他升-苯基丝氨酸衍生物。通过动态控制,2-NO 2-,4-NO 2-,H-和4-CH 4-取代的1-苯基丝氨酸衍生物的de值大于99%syn。突变模型的见解表明,活性中心的结合口袋被重塑了。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号