当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiosulfate enhanced degradation of organic pollutants in aqueous solution with g-C3N4 under visible light irradiation
Chemosphere ( IF 8.1 ) Pub Date : 2021-02-26 , DOI: 10.1016/j.chemosphere.2021.130119 Wenyu Zhang , Chuankun Yin , Yezi Jin , Xianjie Feng , Xiaoxia Li , Aihua Xu
Chemosphere ( IF 8.1 ) Pub Date : 2021-02-26 , DOI: 10.1016/j.chemosphere.2021.130119 Wenyu Zhang , Chuankun Yin , Yezi Jin , Xianjie Feng , Xiaoxia Li , Aihua Xu
|
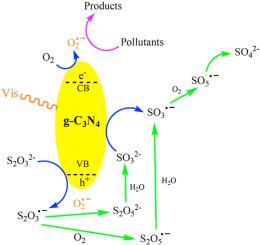
Developing new strategies to design more practicable and efficient g-C3 N4 based photocatalysts is important to solve the environmental issues. Thiosulfate (STS) is a common residual product found in wastewater and removal of STS remains a matter of great environmental concern. In this work, however, STS is activated by g-C3 N4 under visible light irradiation, resulting in a fast degradation of Rhodamine B (RhB) and other pollutants. The performance of g-C3 N4 prepared from urea was much higher than that from melamine, due to the higher surface area and more negative conduction band potential of the former catalyst. In addition, comparison with other oxidants and reductants such as peroxymonosulfate, peroxydisulfate, hydrogen peroxide and sulfite, the use of STS in g-C3 N4 /Vis system showed the highest efficiency for RhB degradation. During ten successive cycles, the excellent reusability of the catalyst was also obtained. The effect of different concentrations of STS and g-C3 N4 , and initial solution pH on the performance of the system were also studied. The mechanism study suggests that STS is first oxidized to S2 O3 − radicals by photohole, which will be transformed to other oxysulfur radicals such as SO3 − and finally to SO4 2− ions. At the same time, the rate of O2 reduction by photoelectrons to O2 − radicals as well as RhB degradation increases. The finding of this study provides a promising advanced oxidation process for organic pollutants degradation via STS activation.
中文翻译:

硫代硫酸盐在可见光照射下用g-C3N4增强水溶液中有机污染物的降解
开发新策略以设计更实用、更高效的 g-C3N4 基光催化剂对于解决环境问题非常重要。硫代硫酸盐 (STS) 是废水中常见的残留产物,去除 STS 仍然是一个非常令人担忧的环境问题。然而,在这项工作中,STS 在可见光照射下被 g-C3N4 激活,导致罗丹明 B (RhB) 和其他污染物快速降解。尿素制备的 g-C3N4 的性能远高于三聚氰胺,因为前者催化剂的表面积更高,负导带电位更大。此外,与其他氧化剂和还原剂如过氧一硫酸盐、过氧二硫酸盐、过氧化氢和亚硫酸盐相比,在 g-C3N4/Vis 系统中使用 STS 显示出最高的 RhB 降解效率。在连续的十次循环中,催化剂也获得了优异的可重用性。还研究了不同浓度的 STS 和 g-C3N4 以及初始溶液 pH 值对系统性能的影响。机理研究表明,STS 首先通过光孔氧化成 S2O3− 自由基,然后转化为其他氧硫自由基,如 SO3−,最后转化为 SO42− 离子。同时,光电子将 O2 还原为 O2− 自由基的速率以及 RhB 降解的速率增加。本研究的结果为通过 STS 活化降解有机污染物提供了一种有前途的高级氧化过程。
更新日期:2021-02-26
中文翻译:

硫代硫酸盐在可见光照射下用g-C3N4增强水溶液中有机污染物的降解
开发新策略以设计更实用、更高效的 g-C3N4 基光催化剂对于解决环境问题非常重要。硫代硫酸盐 (STS) 是废水中常见的残留产物,去除 STS 仍然是一个非常令人担忧的环境问题。然而,在这项工作中,STS 在可见光照射下被 g-C3N4 激活,导致罗丹明 B (RhB) 和其他污染物快速降解。尿素制备的 g-C3N4 的性能远高于三聚氰胺,因为前者催化剂的表面积更高,负导带电位更大。此外,与其他氧化剂和还原剂如过氧一硫酸盐、过氧二硫酸盐、过氧化氢和亚硫酸盐相比,在 g-C3N4/Vis 系统中使用 STS 显示出最高的 RhB 降解效率。在连续的十次循环中,催化剂也获得了优异的可重用性。还研究了不同浓度的 STS 和 g-C3N4 以及初始溶液 pH 值对系统性能的影响。机理研究表明,STS 首先通过光孔氧化成 S2O3− 自由基,然后转化为其他氧硫自由基,如 SO3−,最后转化为 SO42− 离子。同时,光电子将 O2 还原为 O2− 自由基的速率以及 RhB 降解的速率增加。本研究的结果为通过 STS 活化降解有机污染物提供了一种有前途的高级氧化过程。

































 京公网安备 11010802027423号
京公网安备 11010802027423号