Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-02-26 , DOI: 10.1016/j.bmc.2021.116093 Adrianne Wallace-Povirk 1 , Nian Tong 2 , Jennifer Wong-Roushar 3 , Carrie O'Connor 1 , Xilin Zhou 2 , Zhanjun Hou 4 , Xun Bao 1 , Gloria E Garcia 3 , Jing Li 4 , Seongho Kim 4 , Charles E Dann 3 , Larry H Matherly 4 , Aleem Gangjee 2
|
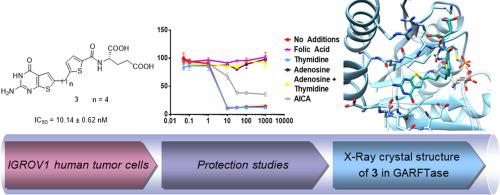
We discovered 6-substituted thieno[2,3-d]pyrimidine compounds (3–9) with 3–4 bridge carbons and side-chain thiophene or furan rings for dual targeting one-carbon (C1) metabolism in folate receptor- (FR) expressing cancers. Synthesis involved nine steps starting from the bromo-aryl carboxylate. From patterns of growth inhibition toward Chinese hamster ovary cells expressing FRα or FRβ, the proton-coupled folate transporter or reduced folate carrier, specificity for uptake by FRs was confirmed. Anti-proliferative activities were demonstrated toward FRα-expressing KB tumor cells and NCI-IGROV1 ovarian cancer cells. Inhibition of de novo purine biosynthesis at both 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase and glycinamide ribonucleotide formyltransferase (GARFTase) was confirmed by metabolite rescue, metabolomics and enzyme assays. X-ray crystallographic structures were obtained with compounds 3–5 and human GARFTase. Our studies identify first-in-class C1 inhibitors with selective uptake by FRs and dual inhibition of enzyme targets in de novo purine biosynthesis, resulting in anti-tumor activity. This series affords an exciting new platform for selective multi-targeted anti-tumor agents.































 京公网安备 11010802027423号
京公网安备 11010802027423号