当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulating the Local Charge Distribution of Ni Active Sites for the Urea Oxidation Reaction
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-02-25 , DOI: 10.1002/anie.202100610 Bo Zhang 1 , Liping Wang 2 , Yajie Zhu 3 , Yunzhou Wen 3 , Shangyu Li 3 , Chunyu Cui 3 , Fenglou Ni 3 , Yunxia Liu 4 , Haiping Lin 4 , Youyong Li 4 , Huisheng Peng 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-02-25 , DOI: 10.1002/anie.202100610 Bo Zhang 1 , Liping Wang 2 , Yajie Zhu 3 , Yunzhou Wen 3 , Shangyu Li 3 , Chunyu Cui 3 , Fenglou Ni 3 , Yunxia Liu 4 , Haiping Lin 4 , Youyong Li 4 , Huisheng Peng 3
Affiliation
|
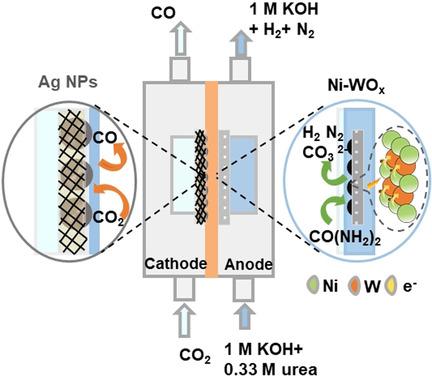
In electrochemical energy storage and conversion systems, the anodic oxygen evolution reaction (OER) accounts for a large proportion of the energy consumption. The electrocatalytic urea oxidation reaction (UOR) is one of the promising alternatives to OER, owing to its low thermodynamic potential. However, owing to the sluggish UOR kinetics, its potential in practical use has not been unlocked. Herein, we developed a tungsten‐doped nickel catalyst (Ni‐WOx) with superior activity towards UOR. The Ni‐WOx catalyst exhibited record fast reaction kinetics (440 mA cm−2 at 1.6 V versus reversible hydrogen electrode) and a high turnover frequency of 0.11 s−1, which is 4.8 times higher than that without W dopants. In further experiments, we found that the W dopant regulated the local charge distribution of Ni atoms, leading to the formation of Ni3+ sites with superior activity and thus accelerating the interfacial catalytic reaction. Moreover, when we integrated Ni‐WOx into a CO2 flow electrolyzer, the cell voltage is reduced to 2.16 V accompanying with ≈98 % Faradaic efficiency towards carbon monoxide.
中文翻译:

调节尿素氧化反应中镍活性位的局部电荷分布
在电化学能量存储和转换系统中,阳极氧放出反应(OER)占了能源消耗的很大一部分。电催化尿素氧化反应(UOR)由于具有较低的热力学潜力,因此是OER的有前途的替代方法之一。但是,由于UOR动力学低迷,其实际应用潜力尚未得到释放。本文中,我们开发了一种对UOR具有优异活性的掺钨镍催化剂(Ni-WO x)。Ni‐WO x催化剂表现出创纪录的快速反应动力学(在1.6 V下与可逆氢电极相比为440 mA cm -2)和0.11 s -1的高周转频率,这是没有掺W的金属的4.8倍。在进一步的实验中,我们发现W掺杂剂可调节Ni原子的局部电荷分布,从而导致具有较高活性的Ni 3+位点的形成,从而加速了界面催化反应。此外,当我们将Ni-WO x集成到CO 2流式电解槽中时,电池电压降至2.16 V,同时对一氧化碳的法拉第效率约为98%。
更新日期:2021-04-27
中文翻译:

调节尿素氧化反应中镍活性位的局部电荷分布
在电化学能量存储和转换系统中,阳极氧放出反应(OER)占了能源消耗的很大一部分。电催化尿素氧化反应(UOR)由于具有较低的热力学潜力,因此是OER的有前途的替代方法之一。但是,由于UOR动力学低迷,其实际应用潜力尚未得到释放。本文中,我们开发了一种对UOR具有优异活性的掺钨镍催化剂(Ni-WO x)。Ni‐WO x催化剂表现出创纪录的快速反应动力学(在1.6 V下与可逆氢电极相比为440 mA cm -2)和0.11 s -1的高周转频率,这是没有掺W的金属的4.8倍。在进一步的实验中,我们发现W掺杂剂可调节Ni原子的局部电荷分布,从而导致具有较高活性的Ni 3+位点的形成,从而加速了界面催化反应。此外,当我们将Ni-WO x集成到CO 2流式电解槽中时,电池电压降至2.16 V,同时对一氧化碳的法拉第效率约为98%。















































 京公网安备 11010802027423号
京公网安备 11010802027423号