European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-02-24 , DOI: 10.1016/j.ejmech.2021.113313 Ran Lu , Yilin Wang , Chunxiao Liu , Zhenguo Zhang , Baiyang Li , Zibo Meng , Cheng Jiang , Qinghua Hu
|
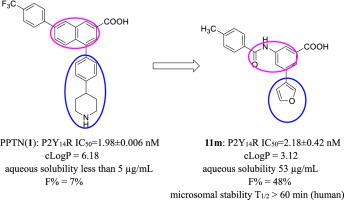
P2Y14 nucleotide receptor plays important roles in series of physiological and pathologic events especially associated with immune and inflammation. Based on the 3-amide benzoic acid scaffold reported by our group previously, a series of 5-aryl-3-amide benzoic acid derivatives were designed as novel P2Y14 antagonists with improved pharmacokinetic properties. Among which compound 11m showed most potent P2Y14 antagonizing activity with an IC50 value of 2.18 nM, furnishing greatly improved water solubility and bioavailability compared with PPTN. In MSU-induced acute gouty arthritis model in mice, 11m exerted promising in vivo efficacy in alleviating mice paw swelling and inflammatory infiltration. Mechanistically, compound 11m notably blocked pyroptosis of macrophages through inhibiting NLRP3 inflammasome activation. This work may contribute to the identification of potential therapeutic agents to intervene in acute gouty arthritis.
中文翻译:

作为新型P2Y 14 R拮抗剂的3-酰胺-5-芳基苯甲酸衍生物的设计,合成和评估,其对急性牙痛性关节炎具有潜在的高效率
P2Y 14核苷酸受体在一系列生理和病理事件中起着重要作用,特别是与免疫和炎症有关。基于我们小组先前报道的3-酰胺苯甲酸支架,设计了一系列5-芳基-3-酰胺苯甲酸衍生物作为具有改善的药代动力学特性的新型P2Y 14拮抗剂。其中化合物11m具有最强的P2Y 14拮抗活性,IC 50值为2.18 nM,与PPTN相比,水溶性和生物利用度大大提高。在MSU诱导的小鼠急性痛风性关节炎模型中,11m在体内发挥了有希望的作用减轻小鼠爪肿胀和炎性浸润的功效。从机理上讲,化合物11m通过抑制NLRP3炎性体的活化来显着阻止巨噬细胞的焦磷酸化。这项工作可能有助于确定干预急性痛风性关节炎的潜在治疗剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号