当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvation structure of the halides from x-ray absorption spectroscopy
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-07-29 15:22:28 , DOI: 10.1063/1.4959589 Matthew Antalek 1 , Elisabetta Pace 2 , Britt Hedman 1 , Keith O Hodgson 3 , Giovanni Chillemi 4 , Maurizio Benfatto 2 , Ritimukta Sarangi 1 , Patrick Frank 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-07-29 15:22:28 , DOI: 10.1063/1.4959589 Matthew Antalek 1 , Elisabetta Pace 2 , Britt Hedman 1 , Keith O Hodgson 3 , Giovanni Chillemi 4 , Maurizio Benfatto 2 , Ritimukta Sarangi 1 , Patrick Frank 1
Affiliation
|
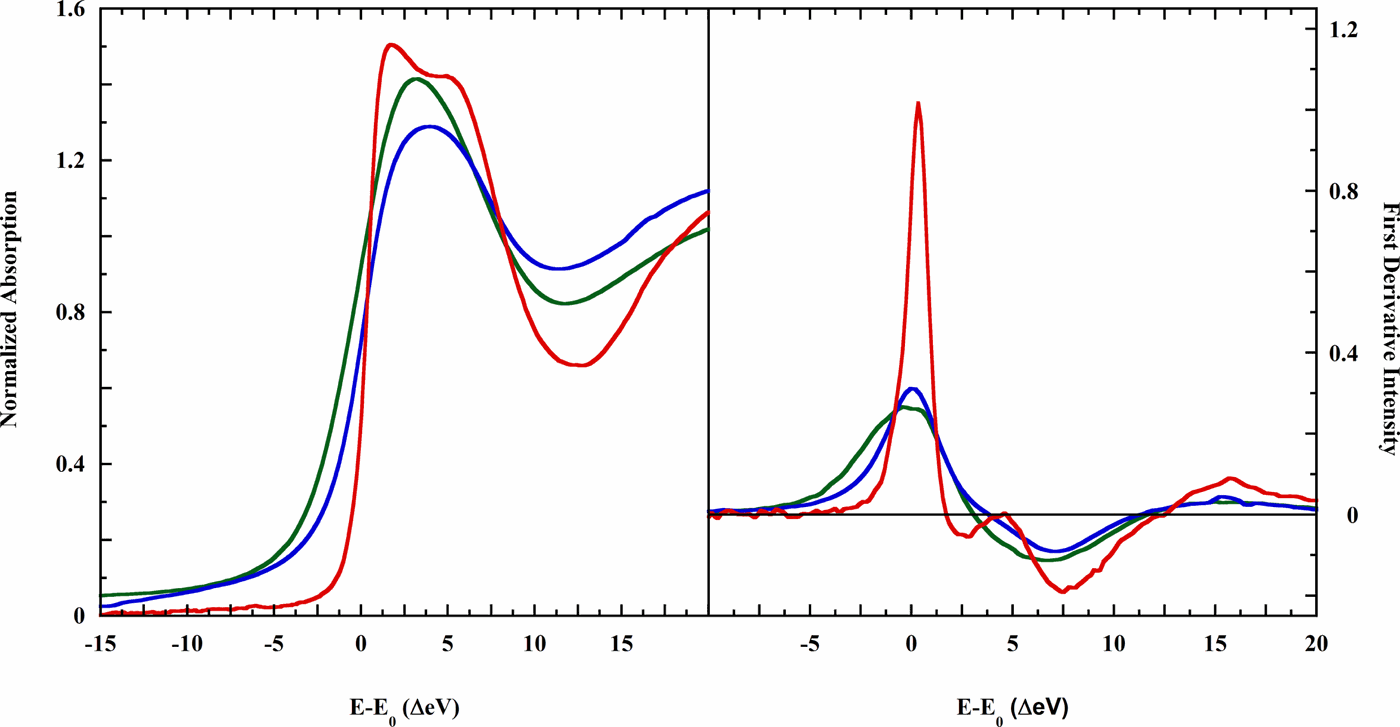
Three-dimensional models for the aqueous solvation structures of chloride, bromide, and iodide are reported. K-edge extended X-ray absorption fine structure (EXAFS) and Minuit X-ray absorption near edge (MXAN) analyses found well-defined single shell solvation spheres for bromide and iodide. However, dissolved chloride proved structurally distinct, with two solvation shells needed to explain its strikingly different X-ray absorption near edge structure (XANES) spectrum. Final solvation models were as follows: iodide, 8 water molecules at 3.60 ± 0.13 Å and bromide, 8 water molecules at 3.40 ± 0.14 Å, while chloride solvation included 7 water molecules at 3.15 ± 0.10 Å, and a second shell of 7 water molecules at 4.14 ± 0.30 Å. Each of the three derived solvation shells is approximately uniformly disposed about the halides, with no global asymmetry. Time-dependent density functional theory calculations simulating the chloride XANES spectra following from alternative solvation spheres revealed surprising sensitivity of the electronic state to 6-, 7-, or 8-coordination, implying a strongly bounded phase space for the correct structure during an MXAN fit. MXAN analysis further showed that the asymmetric solvation predicted from molecular dynamics simulations using halide polarization can play no significant part in bulk solvation. Classical molecular dynamics used to explore chloride solvation found a 7-water solvation shell at 3.12 (−0.04/+0.3) Å, supporting the experimental result. These experiments provide the first fully three-dimensional structures presenting to atomic resolution the aqueous solvation spheres of the larger halide ions.
中文翻译:

X 射线吸收光谱中卤化物的溶剂化结构
报道了氯化物、溴化物和碘化物的水溶剂化结构的三维模型。K 边扩展 X 射线吸收精细结构 (EXAFS) 和 Minuit X 射线吸收近边 (MXAN) 分析发现溴化物和碘化物具有明确的单壳溶剂化球。然而,溶解的氯化物在结构上是不同的,需要两个溶剂化壳层来解释其显着不同的 X 射线吸收近边缘结构 (XANES) 光谱。最终溶剂化模型如下:碘化物,8 个水分子(3.60 ± 0.13 Å)和溴化物,8 个水分子(3.40 ± 0.14 Å),而氯化物溶剂化物包括 7 个水分子(3.15 ± 0.10 Å),以及 7 个水分子的第二层4.14 ± 0.30 埃。三个衍生的溶剂化壳层中的每一个都大致均匀地分布在卤化物周围,没有整体不对称性。时间相关的密度泛函理论计算模拟了替代溶剂化球的氯化物 XANES 光谱,揭示了电子态对 6、7 或 8 配位的惊人敏感性,这意味着 MXAN 拟合期间正确结构的强有界相空间。MXAN 分析进一步表明,使用卤化物极化的分子动力学模拟预测的不对称溶剂化在本体溶剂化中不起重要作用。用于探索氯化物溶剂化的经典分子动力学发现了 3.12 (−0.04/+0.3) Å 处的 7-水溶剂化壳,支持了实验结果。这些实验提供了第一个完整的三维结构,以原子分辨率呈现较大卤化物离子的水溶剂化球。
更新日期:2016-07-30
中文翻译:

X 射线吸收光谱中卤化物的溶剂化结构
报道了氯化物、溴化物和碘化物的水溶剂化结构的三维模型。K 边扩展 X 射线吸收精细结构 (EXAFS) 和 Minuit X 射线吸收近边 (MXAN) 分析发现溴化物和碘化物具有明确的单壳溶剂化球。然而,溶解的氯化物在结构上是不同的,需要两个溶剂化壳层来解释其显着不同的 X 射线吸收近边缘结构 (XANES) 光谱。最终溶剂化模型如下:碘化物,8 个水分子(3.60 ± 0.13 Å)和溴化物,8 个水分子(3.40 ± 0.14 Å),而氯化物溶剂化物包括 7 个水分子(3.15 ± 0.10 Å),以及 7 个水分子的第二层4.14 ± 0.30 埃。三个衍生的溶剂化壳层中的每一个都大致均匀地分布在卤化物周围,没有整体不对称性。时间相关的密度泛函理论计算模拟了替代溶剂化球的氯化物 XANES 光谱,揭示了电子态对 6、7 或 8 配位的惊人敏感性,这意味着 MXAN 拟合期间正确结构的强有界相空间。MXAN 分析进一步表明,使用卤化物极化的分子动力学模拟预测的不对称溶剂化在本体溶剂化中不起重要作用。用于探索氯化物溶剂化的经典分子动力学发现了 3.12 (−0.04/+0.3) Å 处的 7-水溶剂化壳,支持了实验结果。这些实验提供了第一个完整的三维结构,以原子分辨率呈现较大卤化物离子的水溶剂化球。

































 京公网安备 11010802027423号
京公网安备 11010802027423号