Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activated microglia drive demyelination via CSF1R signaling
Glia ( IF 5.4 ) Pub Date : 2021-02-23 , DOI: 10.1002/glia.23980 Dave E Marzan 1, 2 , Valérie Brügger-Verdon 1 , Brian L West 3 , Shane Liddelow 1 , Jayshree Samanta 4 , James L Salzer 1
Glia ( IF 5.4 ) Pub Date : 2021-02-23 , DOI: 10.1002/glia.23980 Dave E Marzan 1, 2 , Valérie Brügger-Verdon 1 , Brian L West 3 , Shane Liddelow 1 , Jayshree Samanta 4 , James L Salzer 1
Affiliation
|
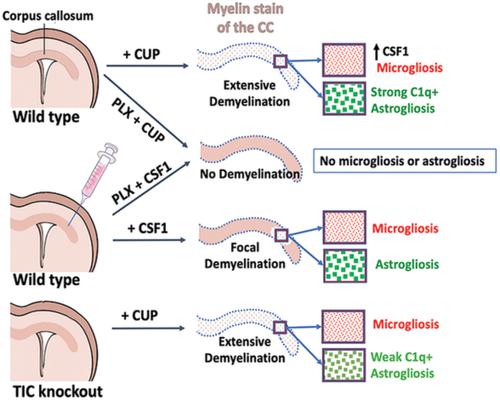
Microgliosis is a prominent pathological feature in many neurological diseases including multiple sclerosis (MS), a progressive auto‐immune demyelinating disorder. The precise role of microglia, parenchymal central nervous system (CNS) macrophages, during demyelination, and the relative contributions of peripheral macrophages are incompletely understood. Classical markers used to identify microglia do not reliably discriminate between microglia and peripheral macrophages, confounding analyses. Here, we use a genetic fate mapping strategy to identify microglia as predominant responders and key effectors of demyelination in the cuprizone (CUP) model. Colony‐stimulating factor 1 (CSF1), also known as macrophage colony‐stimulating factor (M‐CSF) ‐ a secreted cytokine that regulates microglia development and survival—is upregulated in demyelinated white matter lesions. Depletion of microglia with the CSF1R inhibitor PLX3397 greatly abrogates the demyelination, loss of oligodendrocytes, and reactive astrocytosis that results from CUP treatment. Electron microscopy (EM) and serial block face imaging show myelin sheaths remain intact in CUP treated mice depleted of microglia. However, these CUP‐damaged myelin sheaths are lost and robustly phagocytosed upon‐repopulation of microglia. Direct injection of CSF1 into CNS white matter induces focal microgliosis and demyelination indicating active CSF1 signaling can promote demyelination. Finally, mice defective in adopting a toxic astrocyte phenotype that is driven by microglia nevertheless demyelinate normally upon CUP treatment implicating microglia rather than astrocytes as the primary drivers of CUP‐mediated demyelination. Together, these studies indicate activated microglia are required for and can drive demyelination directly and implicate CSF1 signaling in these events.
中文翻译:

激活的小胶质细胞通过 CSF1R 信号驱动脱髓鞘
小胶质细胞增生是许多神经系统疾病的突出病理特征,包括多发性硬化症 (MS),一种进行性自身免疫性脱髓鞘疾病。小胶质细胞、实质中枢神经系统 (CNS) 巨噬细胞在脱髓鞘过程中的确切作用以及外周巨噬细胞的相对贡献尚不完全清楚。用于识别小胶质细胞的经典标记不能可靠地区分小胶质细胞和外周巨噬细胞,混淆分析。在这里,我们使用遗传命运映射策略将小胶质细胞识别为铜宗 (CUP) 模型中脱髓鞘的主要反应者和关键效应者。集落刺激因子 1 (CSF1), 也称为巨噬细胞集落刺激因子 (M-CSF) - 一种调节小胶质细胞发育和存活的分泌细胞因子 - 在脱髓鞘白质病变中上调。用 CSF1R 抑制剂 PLX3397 消除小胶质细胞可大大消除 CUP 治疗导致的脱髓鞘、少突胶质细胞丢失和反应性星形胶质细胞增多症。电子显微镜 (EM) 和连续块状面部成像显示髓鞘在 CUP 处理的小胶质细胞耗尽的小鼠中保持完整。然而,这些受 CUP 损伤的髓鞘在小胶质细胞再生时会丢失并被强烈吞噬。将 CSF1 直接注射到 CNS 白质中会诱导局灶性小胶质细胞增生和脱髓鞘,表明活跃的 CSF1 信号传导可以促进脱髓鞘。最后,小鼠在采用由小胶质细胞驱动的有毒星形胶质细胞表型方面存在缺陷,但在 CUP 治疗后通常会脱髓鞘,这表明小胶质细胞而不是星形胶质细胞是 CUP 介导的脱髓鞘的主要驱动因素。总之,这些研究表明活化的小胶质细胞是脱髓鞘所必需的,并且可以直接驱动脱髓鞘,并将 CSF1 信号传导牵涉到这些事件中。
更新日期:2021-04-09
中文翻译:

激活的小胶质细胞通过 CSF1R 信号驱动脱髓鞘
小胶质细胞增生是许多神经系统疾病的突出病理特征,包括多发性硬化症 (MS),一种进行性自身免疫性脱髓鞘疾病。小胶质细胞、实质中枢神经系统 (CNS) 巨噬细胞在脱髓鞘过程中的确切作用以及外周巨噬细胞的相对贡献尚不完全清楚。用于识别小胶质细胞的经典标记不能可靠地区分小胶质细胞和外周巨噬细胞,混淆分析。在这里,我们使用遗传命运映射策略将小胶质细胞识别为铜宗 (CUP) 模型中脱髓鞘的主要反应者和关键效应者。集落刺激因子 1 (CSF1), 也称为巨噬细胞集落刺激因子 (M-CSF) - 一种调节小胶质细胞发育和存活的分泌细胞因子 - 在脱髓鞘白质病变中上调。用 CSF1R 抑制剂 PLX3397 消除小胶质细胞可大大消除 CUP 治疗导致的脱髓鞘、少突胶质细胞丢失和反应性星形胶质细胞增多症。电子显微镜 (EM) 和连续块状面部成像显示髓鞘在 CUP 处理的小胶质细胞耗尽的小鼠中保持完整。然而,这些受 CUP 损伤的髓鞘在小胶质细胞再生时会丢失并被强烈吞噬。将 CSF1 直接注射到 CNS 白质中会诱导局灶性小胶质细胞增生和脱髓鞘,表明活跃的 CSF1 信号传导可以促进脱髓鞘。最后,小鼠在采用由小胶质细胞驱动的有毒星形胶质细胞表型方面存在缺陷,但在 CUP 治疗后通常会脱髓鞘,这表明小胶质细胞而不是星形胶质细胞是 CUP 介导的脱髓鞘的主要驱动因素。总之,这些研究表明活化的小胶质细胞是脱髓鞘所必需的,并且可以直接驱动脱髓鞘,并将 CSF1 信号传导牵涉到这些事件中。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号