当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization, and Inhibition Study of Novel Substituted Phenylureido Sulfaguanidine Derivatives as α‐Glycosidase and Cholinesterase Inhibitors
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-02-23 , DOI: 10.1002/cbdv.202000958
Suleyman Akocak 1 , Parham Taslimi 2 , Nebih Lolak 1 , Mesut Işık 3 , Mustafa Durgun 4 , Yakup Budak 5 , Cüneyt Türkeş 6 , İlhami Gülçin 7 , Şükrü Beydemir 8, 9
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-02-23 , DOI: 10.1002/cbdv.202000958
Suleyman Akocak 1 , Parham Taslimi 2 , Nebih Lolak 1 , Mesut Işık 3 , Mustafa Durgun 4 , Yakup Budak 5 , Cüneyt Türkeş 6 , İlhami Gülçin 7 , Şükrü Beydemir 8, 9
Affiliation
|
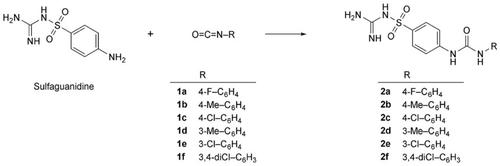
A series of six N‐carbamimidoyl‐4‐(3‐substituted phenylureido)benzenesulfonamide derivatives were synthesized by reaction of sulfaguanidine with aromatic isocyanates. In vitro and in silico inhibitory effects of the novel ureido‐substituted sulfaguanidine derivatives were investigated by spectrophotometric methods for α‐glycosidase (α‐GLY), acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) enzymes associated with diabetes mellitus (DM) and Alzheimer's disease (AD). N‐Carbamimidoyl‐4‐{[(3,4‐dichlorophenyl)carbamoyl]amino}benzene‐1‐sulfonamide (2f) showed AChE and BChE inhibitory effects, with KI values of 515.98±45.03 nM and 598.47±59.18 nM, respectively, while N‐carbamimidoyl‐4‐{[(3‐chlorophenyl)carbamoyl]amino}benzene‐1‐sulfonamide (2e) showed strong α‐GLY inhibitory effect, with KI values of 103.94±13.06 nM. The antidiabetic effects of the novel synthesized compounds are higher than their anti‐Alzheimer's effects, because the inhibition effect of the compounds on the α‐GLY with diabetic enzyme is greater than the effect on esterase enzymes. Indeed, inhibition of the metabolic enzymes is important for the treatment of DM and AD.
中文翻译:

新型取代的苯基脲基磺胺胍衍生物作为α-糖苷酶和胆碱酯酶抑制剂的合成,表征和抑制研究
通过磺胺胍与芳族异氰酸酯的反应合成了一系列六个N-氨基甲酰氨基-4-(3-取代的苯基脲基)苯磺酰胺衍生物。通过分光光度法研究了与糖尿病(DM)和阿尔茨海默氏病有关的α-糖苷酶(α-GLY),乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BChE)酶的新型脲基取代的磺胺胍衍生物的体外和计算机抑制作用。疾病(AD)。N-氨基氨基甲酰基-4-{[((3,4-二氯苯基)氨基甲酰基]氨基}苯-1-磺酰胺(2f)表现出AChE和BChE抑制作用,K I值分别为515.98±45.03 nM和598.47±59.18 nM,而N-氨基甲酰氨基-4-[{((3-氯苯基)氨基甲酰基]氨基}苯-1-磺酰胺(2e)表现出强的α-GLY抑制作用,K I值为103.94±13.06nM。新型合成化合物的抗糖尿病作用高于其抗阿尔茨海默氏症的作用,因为该化合物对糖尿病酶对α-GLY的抑制作用大于对酯酶的抑制作用。实际上,抑制代谢酶对于DM和AD的治疗很重要。
更新日期:2021-04-14
中文翻译:

新型取代的苯基脲基磺胺胍衍生物作为α-糖苷酶和胆碱酯酶抑制剂的合成,表征和抑制研究
通过磺胺胍与芳族异氰酸酯的反应合成了一系列六个N-氨基甲酰氨基-4-(3-取代的苯基脲基)苯磺酰胺衍生物。通过分光光度法研究了与糖尿病(DM)和阿尔茨海默氏病有关的α-糖苷酶(α-GLY),乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BChE)酶的新型脲基取代的磺胺胍衍生物的体外和计算机抑制作用。疾病(AD)。N-氨基氨基甲酰基-4-{[((3,4-二氯苯基)氨基甲酰基]氨基}苯-1-磺酰胺(2f)表现出AChE和BChE抑制作用,K I值分别为515.98±45.03 nM和598.47±59.18 nM,而N-氨基甲酰氨基-4-[{((3-氯苯基)氨基甲酰基]氨基}苯-1-磺酰胺(2e)表现出强的α-GLY抑制作用,K I值为103.94±13.06nM。新型合成化合物的抗糖尿病作用高于其抗阿尔茨海默氏症的作用,因为该化合物对糖尿病酶对α-GLY的抑制作用大于对酯酶的抑制作用。实际上,抑制代谢酶对于DM和AD的治疗很重要。







































 京公网安备 11010802027423号
京公网安备 11010802027423号