Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cooperative Activation of Cellulose with Natural Calcium
JACS Au ( IF 8.5 ) Pub Date : 2021-02-22 , DOI: 10.1021/jacsau.0c00092 Gregory G Facas 1 , Vineet Maliekkal 1 , Cheng Zhu 1 , Matthew Neurock 1 , Paul J Dauenhauer 1
JACS Au ( IF 8.5 ) Pub Date : 2021-02-22 , DOI: 10.1021/jacsau.0c00092 Gregory G Facas 1 , Vineet Maliekkal 1 , Cheng Zhu 1 , Matthew Neurock 1 , Paul J Dauenhauer 1
Affiliation
|
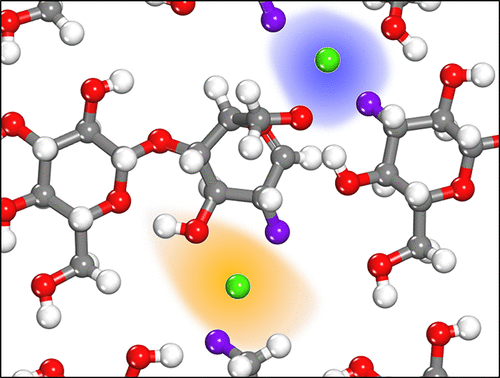
Naturally occurring metals, such as calcium, catalytically activate the intermonomer β-glycosidic bonds in long chains of cellulose, initiating reactions with volatile oxygenates for renewable applications. In this work, the millisecond kinetics of calcium-catalyzed reactions were measured via the method of the pulse-heated analysis of solid and surface reactions (PHASR) at high temperatures (370–430 °C) to reveal accelerated glycosidic ether scission with a second-order rate dependence on the Ca2+ ions. First-principles density functional theory (DFT) calculations were used to identify stable binding configurations for two Ca2+ ions that demonstrated accelerated transglycosylation kinetics, with an apparent activation barrier of 50 kcal mol–1 for a cooperative calcium-catalyzed cycle. The agreement of the mechanism with calcium cooperativity to the experimental barrier (48.7 ± 2.8 kcal mol–1) suggests that calcium enhances the reactivity through a primary role of stabilizing charged transition states and a secondary role of disrupting native H-bonding.
中文翻译:

纤维素与天然钙的协同活化
天然存在的金属,如钙,催化活化纤维素长链中的单体间 β-糖苷键,引发与挥发性含氧化合物的反应,用于可再生应用。在这项工作中,钙催化反应的毫秒动力学是通过高温(370-430°C)下固体和表面反应脉冲加热分析(PHASR)的方法测量的,以揭示加速的糖苷醚断裂级速率依赖于 Ca 2+离子。第一性原理密度泛函理论 (DFT) 计算用于确定两个 Ca 2+离子的稳定结合构型,这些构型表现出加速的转糖基化动力学,表观活化势垒为 50 kcal mol –1用于协同钙催化循环。钙协同机制与实验屏障 (48.7 ± 2.8 kcal mol –1 )的一致性表明,钙通过稳定带电过渡态的主要作用和破坏天然 H 键的次要作用增强了反应性。
更新日期:2021-03-22
中文翻译:

纤维素与天然钙的协同活化
天然存在的金属,如钙,催化活化纤维素长链中的单体间 β-糖苷键,引发与挥发性含氧化合物的反应,用于可再生应用。在这项工作中,钙催化反应的毫秒动力学是通过高温(370-430°C)下固体和表面反应脉冲加热分析(PHASR)的方法测量的,以揭示加速的糖苷醚断裂级速率依赖于 Ca 2+离子。第一性原理密度泛函理论 (DFT) 计算用于确定两个 Ca 2+离子的稳定结合构型,这些构型表现出加速的转糖基化动力学,表观活化势垒为 50 kcal mol –1用于协同钙催化循环。钙协同机制与实验屏障 (48.7 ± 2.8 kcal mol –1 )的一致性表明,钙通过稳定带电过渡态的主要作用和破坏天然 H 键的次要作用增强了反应性。































 京公网安备 11010802027423号
京公网安备 11010802027423号