当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of H+- and Cu+-Sites for N2O Formation during NH3-SCR over Cu-CHA
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-02-22 , DOI: 10.1021/acs.jpcc.0c11008
Yingxin Feng 1 , Ton V. W. Janssens 2 , Peter N. R. Vennestrøm 2 , Jonas Jansson 3 , Magnus Skoglundh 4 , Henrik Grönbeck 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-02-22 , DOI: 10.1021/acs.jpcc.0c11008
Yingxin Feng 1 , Ton V. W. Janssens 2 , Peter N. R. Vennestrøm 2 , Jonas Jansson 3 , Magnus Skoglundh 4 , Henrik Grönbeck 1
Affiliation
|
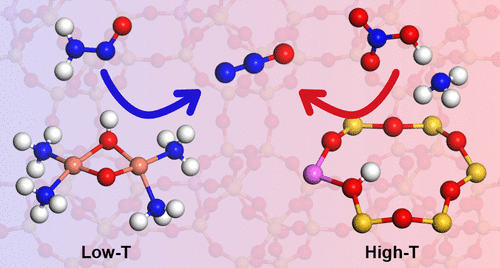
The mechanism for N2O formation over CHA and Cu-CHA zeolite catalysts during NH3-SCR is investigated using density functional theory calculations. Direct NH4NO3 decomposition, which is commonly regarded as the main source of N2O, is found to be associated with high barriers in the absence of Brønsted acid sites. Although Brønsted acid sites promote NH4NO3 decomposition, it is still a highly activated process. Low-temperature N2O formation is instead found to be connected with an NO + NH3 reaction over Cu-sites. In particular, N2O can be formed from H2NNO with a low barrier over Cu-OOH-Cu complexes, which are proposed intermediates in the catalytic cycle for NH3-SCR over Cu-CHA. This finding provides an explanation for the experimentally observed low-temperature N2O formation and the relation between Cu loading and N2O formation. The proposed mechanisms open up strategies to enhance the selectivity to N2 during NH3-SCR.
中文翻译:

Cu + CHA上NH 3 -SCR期间H + -和Cu +-站点在N 2 O形成中的作用。
利用密度泛函理论计算研究了NH 3 -SCR过程中CHA和Cu-CHA沸石催化剂上N 2 O的形成机理。在没有布朗斯台德酸位的情况下,通常被认为是N 2 O的主要来源的直接NH 4 NO 3分解与高势垒有关。尽管布朗斯台德酸中心促进NH 4 NO 3分解,但它仍然是一个高度活化的过程。相反,发现低温N 2 O的形成与Cu位上的NO + NH 3反应有关。特别地,N 2 O可以由H 2形成。在Cu-OOH-Cu络合物上具有低势垒的NNO,它们是NH 3 -SCR在Cu-CHA上催化循环的中间体。该发现为实验观察到的低温N 2 O形成以及Cu负载与N 2 O形成之间的关系提供了解释。所提出的机制开辟了在NH 3 -SCR期间提高对N 2的选择性的策略。
更新日期:2021-03-04
中文翻译:

Cu + CHA上NH 3 -SCR期间H + -和Cu +-站点在N 2 O形成中的作用。
利用密度泛函理论计算研究了NH 3 -SCR过程中CHA和Cu-CHA沸石催化剂上N 2 O的形成机理。在没有布朗斯台德酸位的情况下,通常被认为是N 2 O的主要来源的直接NH 4 NO 3分解与高势垒有关。尽管布朗斯台德酸中心促进NH 4 NO 3分解,但它仍然是一个高度活化的过程。相反,发现低温N 2 O的形成与Cu位上的NO + NH 3反应有关。特别地,N 2 O可以由H 2形成。在Cu-OOH-Cu络合物上具有低势垒的NNO,它们是NH 3 -SCR在Cu-CHA上催化循环的中间体。该发现为实验观察到的低温N 2 O形成以及Cu负载与N 2 O形成之间的关系提供了解释。所提出的机制开辟了在NH 3 -SCR期间提高对N 2的选择性的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号