当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Level Differences in Ionic Solvation and Transport Behavior in Ethylene Oxide-Based Homopolymer and Block Copolymer Electrolytes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-22 , DOI: 10.1021/jacs.0c12538 Daniel Sharon 1, 2 , Peter Bennington 1 , Michael A. Webb 3 , Chuting Deng 1 , Juan J. de Pablo 1, 2 , Shrayesh N. Patel 1 , Paul F. Nealey 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-22 , DOI: 10.1021/jacs.0c12538 Daniel Sharon 1, 2 , Peter Bennington 1 , Michael A. Webb 3 , Chuting Deng 1 , Juan J. de Pablo 1, 2 , Shrayesh N. Patel 1 , Paul F. Nealey 1, 2
Affiliation
|
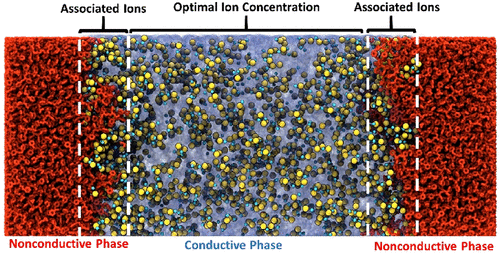
Block copolymer electrolytes (BCE) such as polystyrene-block-poly(ethylene oxide) (SEO) blended with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and composed of mechanically robust insulating and rubbery conducting nanodomains are promising solid-state electrolytes for Li batteries. Here, we compare ionic solvation, association, distribution, and conductivity in SEO-LiTFSI BCEs and their homopolymer PEO-LiTFSI analogs toward a fundamental understanding of the maximum in conductivity and transport mechanisms as a function of salt concentration. Ionic conductivity measurements reveal that SEO-LiTFSI and PEO-LiTFSI exhibit similar behaviors up to a Li/EO ratio of 1/12, where roughly half of the available solvation sites in the system are filled, and conductivity is maximized. As the Li/EO ratios increase to 1/5 the conductivity, of the PEO-LiTFSI drops nearly 3-fold, while the conductivity of SEO-LiTFSI remains constant. FTIR spectroscopy reveals that additional Li cations in the homopolymer electrolyte are complexed by additional EO units when the Li/EO ratio exceeds 1/12, while in the BCE, the proportion of complexed and uncomplexed EO units remains constant; Raman spectroscopy data at the same concentrations show that Li cations in the SEO-LiTFSI samples tend to coordinate more to their counteranions. Atomistic-scale molecular dynamics simulations corroborate these results and further show that associated ions tend to segregate to the SEO-LiTFSI domain interfaces. The opportunity for “excess” salt to be sequestered at BCE interfaces results in the retention of an optimum ratio of uncompleted and complexed PEO solvation sites in the middle of the conductive nanodomains of the BCE and maximized conductivity over a broad range of salt concentrations.
中文翻译:

环氧乙烷基均聚物和嵌段共聚物电解质中离子溶剂化和迁移行为的分子水平差异
嵌段共聚物电解质(BCE),例如聚苯乙烯嵌段与双(三氟甲磺酰基)酰亚胺锂(LiTFSI)混合并由机械强度高的绝缘和橡胶导电纳米域组成的聚环氧乙烷(SEO)是有望用于锂电池的固态电解质。在这里,我们比较了SEO-LiTFSI BCEs及其均聚物PEO-LiTFSI类似物中的离子溶剂化,缔合,分布和电导率,从而对作为盐浓度函数的最大电导率和传输机理有了基本的了解。离子电导率测量表明,SEO-LiTFSI和PEO-LiTFSI在Li / EO比高达1/12时表现出相似的行为,其中系统中约有一半可用的溶剂化位点被填充,并且电导率最大化。当Li / EO之比增加到1/5时,PEO-LiTFSI的电导率下降近3倍,而SEO-LiTFSI的电导率保持恒定。FTIR光谱显示,当Li / EO比超过1/12时,均聚物电解质中的其他Li阳离子会被其他EO单元络合,而在BCE中,络合和未络合的EO单元的比例保持恒定。在相同浓度下的拉曼光谱数据表明,SEO-LiTFSI样品中的Li阳离子倾向于与它们的抗衡阴离子更多地配位。原子尺度的分子动力学模拟证实了这些结果,并进一步表明,相关离子趋于偏向SEO-LiTFSI域界面。
更新日期:2021-03-03
中文翻译:

环氧乙烷基均聚物和嵌段共聚物电解质中离子溶剂化和迁移行为的分子水平差异
嵌段共聚物电解质(BCE),例如聚苯乙烯嵌段与双(三氟甲磺酰基)酰亚胺锂(LiTFSI)混合并由机械强度高的绝缘和橡胶导电纳米域组成的聚环氧乙烷(SEO)是有望用于锂电池的固态电解质。在这里,我们比较了SEO-LiTFSI BCEs及其均聚物PEO-LiTFSI类似物中的离子溶剂化,缔合,分布和电导率,从而对作为盐浓度函数的最大电导率和传输机理有了基本的了解。离子电导率测量表明,SEO-LiTFSI和PEO-LiTFSI在Li / EO比高达1/12时表现出相似的行为,其中系统中约有一半可用的溶剂化位点被填充,并且电导率最大化。当Li / EO之比增加到1/5时,PEO-LiTFSI的电导率下降近3倍,而SEO-LiTFSI的电导率保持恒定。FTIR光谱显示,当Li / EO比超过1/12时,均聚物电解质中的其他Li阳离子会被其他EO单元络合,而在BCE中,络合和未络合的EO单元的比例保持恒定。在相同浓度下的拉曼光谱数据表明,SEO-LiTFSI样品中的Li阳离子倾向于与它们的抗衡阴离子更多地配位。原子尺度的分子动力学模拟证实了这些结果,并进一步表明,相关离子趋于偏向SEO-LiTFSI域界面。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号