当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Computational Study of Asymmetric Hydrogenation of 2-Phenyl Acrylic Acids Catalyzed by a Rh(I) Catalyst with Ferrocenyl Chiral Bisphosphorus Ligand: The Role of Ion-Pair Interaction†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-02-22 , DOI: 10.1002/cjoc.202000741 Xiangru Fan 1 , Lini Zheng 1 , Yuhong Yang 1 , Xiu‐Qin Dong 2 , Xumu Zhang 1 , Lung Wa Chung 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-02-22 , DOI: 10.1002/cjoc.202000741 Xiangru Fan 1 , Lini Zheng 1 , Yuhong Yang 1 , Xiu‐Qin Dong 2 , Xumu Zhang 1 , Lung Wa Chung 1
Affiliation
|
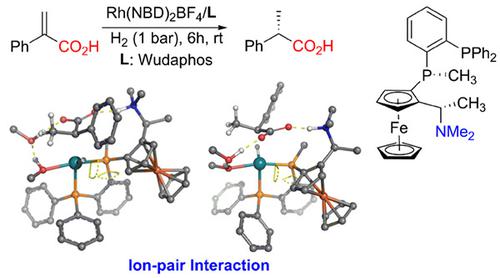
Asymmetric hydrogenation reaction is one of the most efficient synthetic methods to form useful chiral compounds for synthetic chemistry, medicinal chemistry and material chemistry. Generally, the enantioselectivity of many hydrogenation reactions is controlled by steric hindrance between the chiral ligand and substrate. Recently, Zhang group developed a highly asymmetric hydrogenation of 2-aryl and 2-alkyl acrylic acids catalyzed by a Rh(I) catalyst with a chiral Wudaphos ligand. The excellent enantioselectivity of this asymmetric reaction was proposed to be controlled by ion-pair interaction between the substrate and chiral ligand. In this study, a systematic density functional theory study has been carried out to investigate the reaction mechanism and origin of the enantioselectivity. Our computational results suggest that this reaction follows the classic mechanism involving oxidative addition of H2, migratory insertion and reductive elimination. Different from the C=C coordination to the metal in the common oxidative addition step, our study found that the chelation of the carboxyl group of the substrate to the cationic Rh(I) metal is more favorable in this oxidative addition step. The high enantioselectivity is proposed to be dictated by a better catalyst/substrate geometric complementarity in the major pathway to have less distortion of the catalyst for a strong ion-pair interaction. 
中文翻译:

用二茂铁基手性双磷配体的Rh(I)催化剂催化2-苯基丙烯酸不对称加氢的计算研究:离子对相互作用的作用†
不对称氢化反应是形成用于合成化学,药物化学和材料化学的有用手性化合物的最有效的合成方法之一。通常,许多氢化反应的对映选择性是由手性配体和底物之间的位阻控制的。最近,Zhang集团开发了由具有手性Wudaphos配体的Rh(I)催化剂催化的2-芳基和2-烷基丙烯酸的高度不对称氢化反应。提出该不对称反应的优异对映选择性是通过底物和手性配体之间的离子对相互作用来控制的。在这项研究中,已经进行了系统的密度泛函理论研究,以研究对映选择性的反应机理和起源。2,迁移插入和还原消除。我们的研究发现,与普通氧化加成步骤中金属的C = C配位不同,我们发现,在该氧化加成步骤中,底物羧基与阳离子Rh(I)金属的螯合作用更为有利。提出高对映选择性是由主要途径中较好的催化剂/底物几何互补性决定的,以使催化剂具有较小的形变,从而实现强的离子对相互作用。
更新日期:2021-02-22

中文翻译:

用二茂铁基手性双磷配体的Rh(I)催化剂催化2-苯基丙烯酸不对称加氢的计算研究:离子对相互作用的作用†
不对称氢化反应是形成用于合成化学,药物化学和材料化学的有用手性化合物的最有效的合成方法之一。通常,许多氢化反应的对映选择性是由手性配体和底物之间的位阻控制的。最近,Zhang集团开发了由具有手性Wudaphos配体的Rh(I)催化剂催化的2-芳基和2-烷基丙烯酸的高度不对称氢化反应。提出该不对称反应的优异对映选择性是通过底物和手性配体之间的离子对相互作用来控制的。在这项研究中,已经进行了系统的密度泛函理论研究,以研究对映选择性的反应机理和起源。2,迁移插入和还原消除。我们的研究发现,与普通氧化加成步骤中金属的C = C配位不同,我们发现,在该氧化加成步骤中,底物羧基与阳离子Rh(I)金属的螯合作用更为有利。提出高对映选择性是由主要途径中较好的催化剂/底物几何互补性决定的,以使催化剂具有较小的形变,从而实现强的离子对相互作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号