Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2021-02-22 , DOI: 10.1016/j.jacc.2020.12.060 Gentaro Ikeda 1 , Michelle R Santoso 1 , Yuko Tada 1 , Albert M Li 2 , Evgeniya Vaskova 1 , Ji-Hye Jung 1 , Connor O'Brien 1 , Elizabeth Egan 3 , Jiangbin Ye 2 , Phillip C Yang 1
|
Background
Mitochondrial dysfunction results in an imbalance between energy supply and demand in a failing heart. An innovative therapy that targets the intracellular bioenergetics directly through mitochondria transfer may be necessary.
Objectives
The purpose of this study was to establish a preclinical proof-of-concept that extracellular vesicle (EV)-mediated transfer of autologous mitochondria and their related energy source enhance cardiac function through restoration of myocardial bioenergetics.
Methods
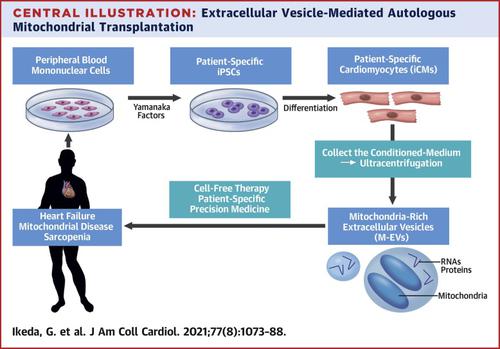
Human-induced pluripotent stem cell–derived cardiomyocytes (iCMs) were employed. iCM-conditioned medium was ultracentrifuged to collect mitochondria-rich EVs (M-EVs). Therapeutic effects of M-EVs were investigated using in vivo murine myocardial infarction (MI) model.
Results
Electron microscopy revealed healthy-shaped mitochondria inside M-EVs. Confocal microscopy showed that M-EV–derived mitochondria were transferred into the recipient iCMs and fused with their endogenous mitochondrial networks. Treatment with 1.0 × 108/ml M-EVs significantly restored the intracellular adenosine triphosphate production and improved contractile profiles of hypoxia-injured iCMs as early as 3 h after treatment. In contrast, isolated mitochondria that contained 300× more mitochondrial proteins than 1.0 × 108/ml M-EVs showed no effect after 24 h. M-EVs contained mitochondrial biogenesis-related messenger ribonucleic acids, including proliferator-activated receptor γ coactivator-1α, which on transfer activated mitochondrial biogenesis in the recipient iCMs at 24 h after treatment. Finally, intramyocardial injection of 1.0 × 108 M-EVs demonstrated significantly improved post-MI cardiac function through restoration of bioenergetics and mitochondrial biogenesis.
Conclusions
M-EVs facilitated immediate transfer of their mitochondrial and nonmitochondrial cargos, contributing to improved intracellular energetics in vitro. Intramyocardial injection of M-EVs enhanced post-MI cardiac function in vivo. This therapy can be developed as a novel, precision therapeutic for mitochondria-related diseases including heart failure.
中文翻译:

来自自体干细胞衍生的心肌细胞的富含线粒体的细胞外囊泡可恢复缺血心肌的能量学
背景
线粒体功能障碍导致心脏衰竭时能量供需失衡。可能需要一种通过线粒体转移直接靶向细胞内生物能量学的创新疗法。
目标
本研究的目的是建立一个临床前概念验证,即细胞外囊泡 (EV) 介导的自体线粒体及其相关能量来源的转移通过恢复心肌生物能量学来增强心脏功能。
方法
使用了人诱导多能干细胞衍生的心肌细胞 (iCM)。iCM 条件培养基经过超速离心以收集富含线粒体的 EV (M-EV)。使用体内小鼠心肌梗塞 (MI) 模型研究了 M-EV 的治疗效果。
结果
电子显微镜显示 M-EV 内部有健康形状的线粒体。共聚焦显微镜显示,M-EV 衍生的线粒体被转移到受体 iCM 中并与其内源性线粒体网络融合。早在处理后 3 小时,用 1.0 × 10 8 /ml M-EVs 处理即可显着恢复细胞内三磷酸腺苷的产生并改善缺氧损伤 iCM 的收缩曲线。相比之下,分离的线粒体所含的线粒体蛋白比 1.0 × 10 8多 300 倍/ml M-EVs 在 24 小时后没有效果。M-EV 含有线粒体生物发生相关的信使核糖核酸,包括增殖物激活受体 γ 辅激活因子-1α,它在处理后 24 小时转移激活受体 iCM 中的线粒体生物发生。最后,心肌内注射 1.0 × 10 8个M-EV 表明,通过恢复生物能量学和线粒体生物发生,显着改善了 MI 后心脏功能。
结论
M-EV 促进了它们的线粒体和非线粒体货物的立即转移,有助于改善体外细胞内能量学。心肌内注射 M-EVs 可增强体内 MI 后心脏功能。这种疗法可以开发为一种新颖的、精确的疗法,用于治疗包括心力衰竭在内的线粒体相关疾病。















































 京公网安备 11010802027423号
京公网安备 11010802027423号