Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2021-02-22 , DOI: 10.1016/j.apcatb.2021.120030 Xiaolong Zhang , Chuangwei Liu , Yong Zhao , Linbo Li , Yu Chen , Fazal Raziq , Liang Qiao , Si-Xuan Guo , Caiyun Wang , Gordon G. Wallace , Alan M. Bond , Jie Zhang
|
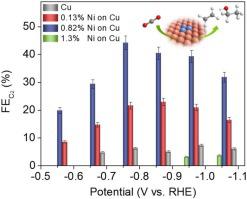
This work describes a coordination enabled galvanic replacement method to decorate atomic Ni clusters on defect-rich Cu surface to provide the first Ni/Cu bimetallic system that significantly enhances the production of C2 products from electrocatalytic CO2 reduction. Specifically, with a surface Ni/Cu ratio of 0.82 %, a 7-fold increase in the selectivity for C2 products was found in comparison with pristine Cu. Density functional theory calculations reveal that the rate determining step for *CO formation changes from the formation of *COOH on copper to the chemisorption of CO2 on Ni decorated surfaces. An alteration of binding sites from Ni-Ni bridge for *CO2 and *COOH to Ni-Cu bridge for *CO is discovered and is proposed to favor the key C C coupling step. The catalytic mechanism demonstrated in the Cu-Ni system points to the new directions for the development of advanced bimetallic electrocatalysts for producing multi-carbon materials from CO2 reduction.
C coupling step. The catalytic mechanism demonstrated in the Cu-Ni system points to the new directions for the development of advanced bimetallic electrocatalysts for producing multi-carbon materials from CO2 reduction.
中文翻译:

原子镍簇装饰的富缺陷铜可提高电催化还原CO 2的C 2产物选择性
这项工作描述了一种能使配位的电置换方法来装饰富含缺陷的Cu表面上的原子Ni团簇,从而提供了第一个Ni / Cu双金属系统,该系统显着提高了电催化还原CO 2产生的C 2产物的能力。具体而言,在表面Ni / Cu比为0.82%的情况下,与原始Cu相比,发现C 2产物的选择性提高了7倍。密度泛函理论计算表明,* CO形成的速率确定步骤从铜上* COOH的形成到Ni装饰表面上化学吸附CO 2的过程有所不同。Ni-Ni桥对* CO 2的结合位点的变化并发现了* COOH到* CO的Ni-Cu桥,并被提议用于关键的C  C偶联步骤。Cu-Ni体系中显示的催化机理为先进的双金属电催化剂的开发指明了新的方向,该双金属电催化剂可通过还原CO 2来生产多碳材料。
C偶联步骤。Cu-Ni体系中显示的催化机理为先进的双金属电催化剂的开发指明了新的方向,该双金属电催化剂可通过还原CO 2来生产多碳材料。

































 京公网安备 11010802027423号
京公网安备 11010802027423号