当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward Practical High‐Areal‐Capacity Aqueous Zinc‐Metal Batteries: Quantifying Hydrogen Evolution and a Solid‐Ion Conductor for Stable Zinc Anodes
Advanced Materials ( IF 27.4 ) Pub Date : 2021-02-19 , DOI: 10.1002/adma.202007406 Longtao Ma 1 , Qing Li 1 , Yiran Ying 2 , Feixiang Ma 1 , Shengmei Chen 1 , Yangyang Li 1 , Haitao Huang 2 , Chunyi Zhi 1, 3
Advanced Materials ( IF 27.4 ) Pub Date : 2021-02-19 , DOI: 10.1002/adma.202007406 Longtao Ma 1 , Qing Li 1 , Yiran Ying 2 , Feixiang Ma 1 , Shengmei Chen 1 , Yangyang Li 1 , Haitao Huang 2 , Chunyi Zhi 1, 3
Affiliation
|
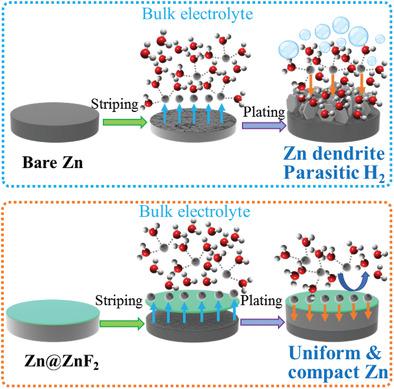
The hydrogen evolution in Zn metal battery is accurately quantified by in situ battery–gas chromatography–mass analysis. The hydrogen fluxes reach 3.76 mmol h−1 cm−2 in a Zn//Zn symmetric cell in each segment, and 7.70 mmol h−1 cm−2 in a Zn//MnO2 full cell. Then, a highly electronically insulating (0.11 mS cm−1) but highly Zn2+ ion conductive (80.2 mS cm−1) ZnF2 solid ion conductor with high Zn2+ transfer number (0.65) is constructed to isolate Zn metal from liquid electrolyte, which not only prohibits over 99.2% parasitic hydrogen evolution but also guides uniform Zn electrodeposition. Precisely quantitated, the Zn@ZnF2//Zn@ZnF2 cell only produces 0.02 mmol h−1 cm−2 of hydrogen (0.53% of the Zn//Zn cell). Encouragingly, a high‐areal‐capacity Zn@ZnF2//MnO2 (≈3.2 mAh cm−2) full cell only produces maximum hydrogen flux of 0.06 mmol h−1 cm−2 (0.78% of the Zn//Zn cell) at the fully charging state. Meanwhile, Zn@ZnF2//Zn@ZnF2 symmetric cell exhibits excellent stability under ultrahigh current density and areal capacity (10 mA cm−2, 10 mAh cm−2) over 590 h (285 cycles), which far outperforms all reported Zn metal anodes in aqueous systems. In light of the superior Zn@ZnF2 anode, the high‐areal‐capacity aqueous Zn@ZnF2//MnO2 batteries (≈3.2 mAh cm−2) shows remarkable cycling stability over 1000 cycles with 93.63% capacity retained at ≈100% Coulombic efficiency.
中文翻译:

迈向实用的高容量锌金属水溶液电池:量化氢的逸出量和用于稳定锌阳极的固体离子导体
通过原位电池-气相色谱-质谱分析可准确定量锌金属电池中的氢气释放量。在每个分段的Zn // Zn对称电池中,氢通量达到3.76 mmol h -1 cm -2,而在Zn // MnO 2充满电池中,氢通量达到7.70 mmol h -1 cm -2。然后,形成具有高电子绝缘性(0.11 mS cm -1)但具有高Zn 2+离子导电性(80.2 mS cm -1)ZnF 2固体离子导体和Zn 2+转移数(0.65)用于将锌金属与液体电解质隔离,这不仅可以抑制99.2%以上的寄生氢放出,还可以指导均匀的Zn电沉积。精确定量后,Zn @ ZnF 2 // Zn @ ZnF 2池仅产生0.02 mmol h -1 cm -2的氢气(占Zn // Zn池的0.53%)。令人鼓舞的是,高面积容量的Zn @ ZnF 2 // MnO 2(≈3.2mAh cm -2)满电池只能产生0.06 mmol h -1 cm -2的最大氢通量(占Zn // Zn电池的0.78% )处于完全充电状态。同时,Zn @ ZnF 2 // Zn @ ZnF 2对称电池在590小时(285次循环)中,在超高电流密度和面容量(10 mA cm -2,10 mAh cm -2)下表现出出色的稳定性,远胜过水性系统中所有报道的Zn金属阳极。根据出色的Zn @ ZnF 2阳极,高容量的Zn @ ZnF 2 // MnO 2水溶液(≈3.2mAh cm -2)在1000次循环中显示出显着的循环稳定性,在≈100时保持93.63%的容量库仑效率%。
更新日期:2021-03-23
中文翻译:

迈向实用的高容量锌金属水溶液电池:量化氢的逸出量和用于稳定锌阳极的固体离子导体
通过原位电池-气相色谱-质谱分析可准确定量锌金属电池中的氢气释放量。在每个分段的Zn // Zn对称电池中,氢通量达到3.76 mmol h -1 cm -2,而在Zn // MnO 2充满电池中,氢通量达到7.70 mmol h -1 cm -2。然后,形成具有高电子绝缘性(0.11 mS cm -1)但具有高Zn 2+离子导电性(80.2 mS cm -1)ZnF 2固体离子导体和Zn 2+转移数(0.65)用于将锌金属与液体电解质隔离,这不仅可以抑制99.2%以上的寄生氢放出,还可以指导均匀的Zn电沉积。精确定量后,Zn @ ZnF 2 // Zn @ ZnF 2池仅产生0.02 mmol h -1 cm -2的氢气(占Zn // Zn池的0.53%)。令人鼓舞的是,高面积容量的Zn @ ZnF 2 // MnO 2(≈3.2mAh cm -2)满电池只能产生0.06 mmol h -1 cm -2的最大氢通量(占Zn // Zn电池的0.78% )处于完全充电状态。同时,Zn @ ZnF 2 // Zn @ ZnF 2对称电池在590小时(285次循环)中,在超高电流密度和面容量(10 mA cm -2,10 mAh cm -2)下表现出出色的稳定性,远胜过水性系统中所有报道的Zn金属阳极。根据出色的Zn @ ZnF 2阳极,高容量的Zn @ ZnF 2 // MnO 2水溶液(≈3.2mAh cm -2)在1000次循环中显示出显着的循环稳定性,在≈100时保持93.63%的容量库仑效率%。































 京公网安备 11010802027423号
京公网安备 11010802027423号