当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intracellular Mutual Promotion of Redox Homeostasis Regulation and Iron Metabolism Disruption for Enduring Chemodynamic Therapy
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-02-19 , DOI: 10.1002/adfm.202010390 Yang Liu 1 , Shaojie Zhai 2 , Xingwu Jiang 3, 4 , Yanyan Liu 3 , Kun Wang 1 , Chaochao Wang 4 , Meng Zhang 2 , Xuanyong Liu 2 , Wenbo Bu 1, 2, 3
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-02-19 , DOI: 10.1002/adfm.202010390 Yang Liu 1 , Shaojie Zhai 2 , Xingwu Jiang 3, 4 , Yanyan Liu 3 , Kun Wang 1 , Chaochao Wang 4 , Meng Zhang 2 , Xuanyong Liu 2 , Wenbo Bu 1, 2, 3
Affiliation
|
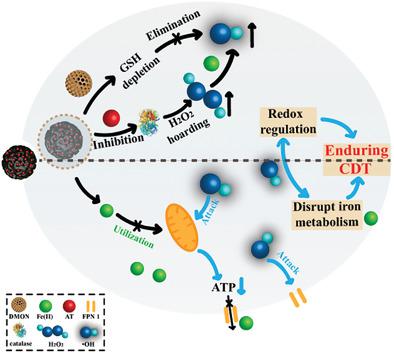
Intracellular redox homeostasis and the iron metabolism system in tumor cells are closely associated with the limited efficacy of chemodynamic therapy (CDT). Despite extensive attempts, maintaining high levels of intracellular catalysts (free iron) and reactants (H2O2) while decreasing the content of reactive oxygen species (ROS) scavengers (especially glutathione (GSH)) for enduring CDT still remains great challenges. Herein, SS bond‐rich dendritic mesoporous organic silica nanoparticles (DMON) are utilized as GSH‐depleting agents. After co‐loading Fe0 and a catalase inhibitor (3‐amino‐1,2,4‐triazole (AT)), a novel biodegradable nanocarrier is constructed as DMON@Fe0/AT. In the mildly acidic tumor microenvironment, on‐demand ferrous ions and AT are intelligently released. AT suppresses the activity of catalase for H2O2 hoarding, and the exposed DMON weakens ROS scavenging systems by persistently depleting intracellular GSH. The highly efficient •OH production by DMON@Fe0/AT can effectively attack mitochondria and downregulate the expression of ferroportin 1, which can disrupt the cellular iron metabolism system, leading to the desired retention of iron in the cytoplasm. More importantly, DMON@Fe0/AT exhibits a much more efficient CDT killing effect on 4T1 tumor cells than plain Fe0 nanoparticles, benefiting from their synergistic redox regulation and iron metabolism disruption. Overall, the as‐prepared intelligent, degradable DMON@Fe0/AT provides an innovative strategy for enduring CDT.
中文翻译:

细胞内相互促进氧化还原稳态调节和铁代谢破坏持久的化学动力疗法。
细胞内氧化还原稳态和肿瘤细胞中的铁代谢系统与化学动力疗法(CDT)的有限疗效密切相关。尽管进行了广泛的尝试,但保持高水平的细胞内催化剂(游离铁)和反应物(H 2 O 2),同时降低持久性CDT的活性氧(ROS)清除剂(尤其是谷胱甘肽(GSH))的含量仍然是巨大的挑战。在本文中,富含SS键的树枝状介孔有机二氧化硅纳米粒子(DMON)被用作GSH消耗剂。将Fe 0和过氧化氢酶抑制剂(3-氨基1,2,4-三唑(AT))共同负载后,构建了一种新型的可生物降解的纳米载体DMON @ Fe 0/在。在弱酸性的肿瘤微环境中,智能地释放了按需的亚铁离子和AT。AT抑制H 2 O 2 H积的过氧化氢酶活性,而暴露的DMON通过持续消耗细胞内GSH来削弱ROS清除系统。DMON @ Fe 0 / AT产生的高效•OH可以有效攻击线粒体并下调Ferroportin 1的表达,从而破坏细胞铁代谢系统,从而使铁在细胞质中保持所需的保留率。更重要的是,DMON @ Fe 0 / AT对4T1肿瘤细胞的CDT杀伤作用比普通Fe 0更为有效。纳米颗粒,得益于其协同的氧化还原调节和铁代谢破坏。总体而言,准备好的智能可降解DMON @ Fe 0 / AT提供了一种持久CDT的创新策略。
更新日期:2021-04-22
中文翻译:

细胞内相互促进氧化还原稳态调节和铁代谢破坏持久的化学动力疗法。
细胞内氧化还原稳态和肿瘤细胞中的铁代谢系统与化学动力疗法(CDT)的有限疗效密切相关。尽管进行了广泛的尝试,但保持高水平的细胞内催化剂(游离铁)和反应物(H 2 O 2),同时降低持久性CDT的活性氧(ROS)清除剂(尤其是谷胱甘肽(GSH))的含量仍然是巨大的挑战。在本文中,富含SS键的树枝状介孔有机二氧化硅纳米粒子(DMON)被用作GSH消耗剂。将Fe 0和过氧化氢酶抑制剂(3-氨基1,2,4-三唑(AT))共同负载后,构建了一种新型的可生物降解的纳米载体DMON @ Fe 0/在。在弱酸性的肿瘤微环境中,智能地释放了按需的亚铁离子和AT。AT抑制H 2 O 2 H积的过氧化氢酶活性,而暴露的DMON通过持续消耗细胞内GSH来削弱ROS清除系统。DMON @ Fe 0 / AT产生的高效•OH可以有效攻击线粒体并下调Ferroportin 1的表达,从而破坏细胞铁代谢系统,从而使铁在细胞质中保持所需的保留率。更重要的是,DMON @ Fe 0 / AT对4T1肿瘤细胞的CDT杀伤作用比普通Fe 0更为有效。纳米颗粒,得益于其协同的氧化还原调节和铁代谢破坏。总体而言,准备好的智能可降解DMON @ Fe 0 / AT提供了一种持久CDT的创新策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号