Biomaterials ( IF 12.8 ) Pub Date : 2021-02-18 , DOI: 10.1016/j.biomaterials.2021.120726 Wenjing Li , Zhe Jing , Shuqing Wang , Qiyu Li , Yutong Xing , Haobo Shi , Shuang Li , Zhangyong Hong
|
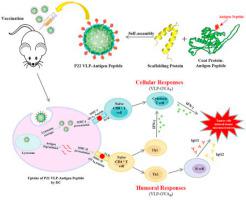
As a new strategy for cancer immunotherapy, therapeutic cancer vaccines have been greatly improved in recent years. However, addressing the needs to quickly and efficiently elicit a high-intensity immune response against neoantigen peptides, especially to induce an effective cytotoxic lymphocyte (CTL) reaction, remain challenges in this field. In this study, virus-like particles (VLPs) derived from the phage P22 were adopted to load peptide antigens on the surface, to test whether VLP technology can be used as a platform for efficient peptide antigen delivery by therapeutic cancer vaccines. The B and T epitopes (OVAB peptide and OVAT peptide) of ovalbumin (OVA) were used here as model antigens and fused individually at the C terminus of the coat protein (CP), which allowed display on the surface of P22 particles to form two types of vaccine particles (VLP-OVAB and VLP-OVAT). Subsequent experiments showed that VLP-OVAB induced an antibody titer against the peptide antigen as high as 5.0 × 105 and that VLP-OVAT induced highly effective cross-presentation and then strongly activated a T epitope-specific CTL response. Mouse tumor model experiments showed that VLP-OVAT could significantly inhibit tumor growth by increasing the proportions of CD4+ T cells, CD8+ T cells and effector memory T cells (TEM cells) and lowering the proportion of myeloid-derived suppressor cells (MDSCs) among tumor-infiltrating lymphocytes and splenocytes. Compared with other chemically synthesized nanomaterials, VLPs have obvious advantages as vaccine carriers due to their clear chemical composition, fixed spatial structure, excellent biocompatibility, and relatively high potential for clinical translation. Therefore, this platform may lay a solid foundation for the design and preparation of personalized therapeutic vaccines based on neoantigen peptides.
中文翻译:

P22病毒样颗粒作为用于癌症免疫治疗的有效抗原递送纳米平台
作为癌症免疫疗法的一种新策略,治疗性癌症疫苗近年来已得到了极大的改进。然而,解决快速有效地引发针对新抗原肽的高强度免疫应答,尤其是诱导有效的细胞毒性淋巴细胞(CTL)反应的需求,仍然是该领域的挑战。在这项研究中,采用源自噬菌体P22的病毒样颗粒(VLP)将肽抗原负载在表面上,以测试VLP技术是否可以用作治疗性癌症疫苗高效肽抗原递送的平台。卵清蛋白的B和T表位(OVA B肽和OVA T肽)(OVA)在此处用作模型抗原,并分别在外壳蛋白(CP)的C末端融合,从而可以在P22颗粒的表面展示以形成两种类型的疫苗颗粒(VLP-OVA B和VLP-OVA T)。随后的实验表明,VLP-OVA B诱导的针对肽抗原的抗体效价高达5.0×10 5,并且VLP-OVA T诱导了高效的交叉呈递,然后强烈激活了T表位特异性CTL反应。小鼠肿瘤模型实验表明,VLP-OVA T可通过增加CD4 + T细胞,CD8 +的比例来显着抑制肿瘤生长T细胞和效应记忆T细胞(T EM细胞),并降低了肿瘤浸润淋巴细胞和脾细胞中髓样抑制细胞(MDSC)的比例。与其他化学合成的纳米材料相比,VLP由于其清晰的化学组成,固定的空间结构,出色的生物相容性和相对较高的临床翻译潜力,具有作为疫苗载体的明显优势。因此,该平台可为基于新抗原肽的个性化治疗疫苗的设计和制备奠定坚实的基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号