European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-02-18 , DOI: 10.1016/j.ejmech.2021.113299 Václav Němec , Lukáš Maier , Benedict-Tilman Berger , Apirat Chaikuad , Stanislav Drápela , Karel Souček , Stefan Knapp , Kamil Paruch
|
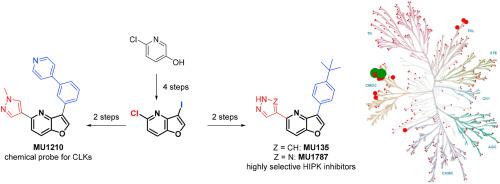
The furo [3,2-b]pyridine motif represents a relatively underexplored central pharmacophore in the area of kinase inhibitors. Herein, we report flexible synthesis of 3,5-disubstituted furo [3,2-b]pyridines that relies on chemoselective couplings of newly prepared 5-chloro-3-iodofuro [3,2-b]pyridine. This methodology allowed efficient second-generation synthesis of the state-of-the-art chemical biology probe for CLK1/2/4 MU1210, and identification of the highly selective inhibitors of HIPKs MU135 and MU1787 which are presented and characterized in this study, including the X-ray crystal structure of MU135 in HIPK2.
chemical biology probe
中文翻译:

具有呋喃[3,2- b ]吡啶核的蛋白激酶CLK和HIPK的高选择性抑制剂
呋喃[3,2- b ]吡啶基序代表激酶抑制剂领域相对未开发的中心药效基团。在本文中,我们报道了3,5-二取代的呋喃并[3,2-的柔性合成b ]吡啶,它依赖于新制备的5-氯-3-碘呋喃并[3,2-的化学选择性耦合b ]吡啶。这种方法可以对第二代CLK1 / 2/4 MU1210的最新化学生物学探针进行有效的第二代合成,并鉴定出本研究中介绍和表征的HIPK MU135和MU1787的高选择性抑制剂,包括HIPK2中MU135的X射线晶体结构。
化学生物学探针




















































 京公网安备 11010802027423号
京公网安备 11010802027423号