Carbon ( IF 10.5 ) Pub Date : 2021-02-18 , DOI: 10.1016/j.carbon.2021.02.053
Hector Gomez , Michael N. Groves
|
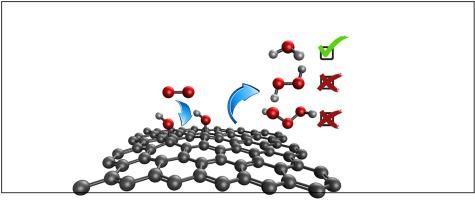
The role of gas on graphene oxide (GO) surfaces is an area of great interest for heterogeneous catalytic materials in ambient and interstellar conditions. As a result, we investigated the transfer mechanisms of surface hydrogens from OH groups on the basal plane of GO using density functional theory. The reaction mechanisms were calculated in both triplet and singlet states due to the ground state spin states of and the products. By passing gas over GO, we found , , and (singlet state) were generated products. In reference to triplet , we found that was produced exothermically, with differences in energy of −0.423 eV and −0.048 eV in the triplet and singlet states, respectively. The triplet state is favored in formation since the acted as a hydrogen shuttle and transported an H-atom from one group to the other. and formation occurred endothermically, requiring 1.08 eV and 0.978 eV in the singlet state, respectively. Additionally, in the triplet state resulted in a net energy difference of 2.22 eV. Based on these calculations, GO is a highly unfavorable catalyst for formation on the basal plane and is thermodynamically inclined to form .
中文翻译:

氧化石墨烯(GO)表面上H 2 O,H 2 O 2和H 2 O 3形成机理的建模
的作用 氧化石墨烯(GO)表面上的气体是环境和星际条件下非均相催化材料的重要研究领域。结果,我们使用密度泛函理论研究了GO基面上OH基表面氢的转移机理。由于三价态和三价态的基态自旋态,计算了反应机理。和产品。通过传递 在GO上加油,我们发现 , , 和 (单一状态)生成的产品。关于三胞胎,我们发现 在三重态和单重态下,其放热产生的能量差分别为-0.423 eV和-0.048 eV。三重态在 自 充当氢穿梭机并从一个氢原子运来一个H原子 分组到另一个。 和 吸热形成,在单重态分别需要1.08 eV和0.978 eV。此外,在三重态下的净能量差为2.22 eV。根据这些计算,GO是极不利的催化剂 在基面上形成并热力学倾斜形成 。

































 京公网安备 11010802027423号
京公网安备 11010802027423号