当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Nickel-Based Single-Atom Alloy Catalyst for CO2 Conversion Reactions: Computational Screening and Reaction Mechanism Analysis
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-02-16 , DOI: 10.1021/acs.jpcc.0c11578 Ojus Mohan 1, 2, 3 , Rong Xu 2 , Samir H. Mushrif 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-02-16 , DOI: 10.1021/acs.jpcc.0c11578 Ojus Mohan 1, 2, 3 , Rong Xu 2 , Samir H. Mushrif 3
Affiliation
|
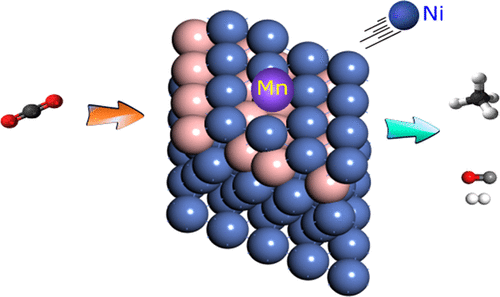
Catalytic conversion of CO2 to methane and syngas are two promising routes for CO2 utilization. Even though noble metals have shown high activity and stability for these reactions, cheaper nickel (Ni)-based catalysts are preferred. However, these are less active and are prone to deactivation due to carbon deposition. Boron-promoted Ni (NiB) was developed by the microstructural modification of Ni, and it showed improved stability, but with reduced activity. This is attributed to the high CO2 activation barrier. Single-atom alloys (SAA) are adequately capable of performing selective catalysis to improve the catalyst performance. In this work, using first-principles-based computational screening, a novel and thermodynamically stable NiB SAA catalyst that reduces CO2 activation barrier and improves the activity without affecting stability and selectivity of NiB is predicted. We considered 14 dopant elements that alloy with Ni (Ru, Pt, Pd, Rh, Co, Fe, Os, Ir, Re, W, Mo, Cu, Mn, and Zn) and evaluated the relative thermodynamic stability of the SAA configuration, compared to that of dimer or trimer structures. The relative stability of SAA was higher than that of the aggregates for only five metals (Pt, Pd, Rh, Cu, and Mn). We further calculated the CO2 activation barrier on these five SAAs and found that Mn-NiB SAA was the only candidate on which there is a significant reduction of the CO2 activation barrier (reduced by 56 kJ mol–1). Subsequently, we studied CO2 methanation (46 elementary reactions) and dry reforming of methane (DRM) (38 elementary reactions) reactions on Mn-NiB SAA. High CO2 adsorption energy and low CO2 and CO* activation barriers make Mn-NiB SAA a suitable catalyst for CO2 methanation. Correspondingly, the low CO2 and CH4 activation barriers make Mn-NiB SAA a perfect candidate for DRM reaction. Prominently, the high endergonicity for CH4 stepwise dehydrogenation combined with a low barrier for Boudouard reaction reduces the on-surface coke formation. Thus, we believe that Mn-NiB SAA can be a potential catalyst for CO2 methanation and DRM reactions.
中文翻译:

用于CO 2转化反应的新型镍基单原子合金催化剂:计算筛选和反应机理分析
CO 2催化转化为甲烷和合成气是利用CO 2的两个有希望的途径。即使贵金属已显示出对于这些反应的高活性和稳定性,更便宜的镍(Ni)基催化剂是优选的。然而,这些活性较低,并且由于碳沉积而易于失活。硼促进的镍(NiB)是通过镍的微结构改性而开发的,它显示出改善的稳定性,但活性降低。这归因于高的CO 2激活屏障。单原子合金(SAA)足以执行选择性催化以改善催化剂性能。在这项工作中,使用基于第一原理的计算筛选技术,可以预测出一种新型且热力学稳定的NiB SAA催化剂,该催化剂可降低CO 2活化势垒并提高活性,而不会影响NiB的稳定性和选择性。我们考虑了14种与Ni形成合金的掺杂元素(Ru,Pt,Pd,Rh,Co,Fe,Os,Ir,Re,W,Mo,Cu,Mn和Zn),并评估了SAA构型的相对热力学稳定性,与二聚体或三聚体结构相比。对于仅五种金属(Pt,Pd,Rh,Cu和Mn),SAA的相对稳定性高于聚集体。我们进一步计算了CO 2这五个SAA上的活化障碍,发现Mn-NiB SAA是唯一显着降低CO 2活化障碍(降低56 kJ mol –1)的候选物质。随后,我们研究了在Mn-NiB SAA上进行CO 2甲烷化(46个基本反应)和甲烷干重整(DRM)(38个基本反应)反应。高的CO 2吸附能和低的CO 2和CO *活化势垒使Mn-NiB SAA成为适合CO 2甲烷化的催化剂。相应地,低的CO 2和CH 4活化势垒使Mn-NiB SAA成为DRM反应的理想选择。突出的是,CH 4的高发光度逐步脱氢结合用于Boudouard反应的低阻隔层可减少表面焦炭的形成。因此,我们认为Mn-NiB SAA可以作为CO 2甲烷化和DRM反应的潜在催化剂。
更新日期:2021-02-25
中文翻译:

用于CO 2转化反应的新型镍基单原子合金催化剂:计算筛选和反应机理分析
CO 2催化转化为甲烷和合成气是利用CO 2的两个有希望的途径。即使贵金属已显示出对于这些反应的高活性和稳定性,更便宜的镍(Ni)基催化剂是优选的。然而,这些活性较低,并且由于碳沉积而易于失活。硼促进的镍(NiB)是通过镍的微结构改性而开发的,它显示出改善的稳定性,但活性降低。这归因于高的CO 2激活屏障。单原子合金(SAA)足以执行选择性催化以改善催化剂性能。在这项工作中,使用基于第一原理的计算筛选技术,可以预测出一种新型且热力学稳定的NiB SAA催化剂,该催化剂可降低CO 2活化势垒并提高活性,而不会影响NiB的稳定性和选择性。我们考虑了14种与Ni形成合金的掺杂元素(Ru,Pt,Pd,Rh,Co,Fe,Os,Ir,Re,W,Mo,Cu,Mn和Zn),并评估了SAA构型的相对热力学稳定性,与二聚体或三聚体结构相比。对于仅五种金属(Pt,Pd,Rh,Cu和Mn),SAA的相对稳定性高于聚集体。我们进一步计算了CO 2这五个SAA上的活化障碍,发现Mn-NiB SAA是唯一显着降低CO 2活化障碍(降低56 kJ mol –1)的候选物质。随后,我们研究了在Mn-NiB SAA上进行CO 2甲烷化(46个基本反应)和甲烷干重整(DRM)(38个基本反应)反应。高的CO 2吸附能和低的CO 2和CO *活化势垒使Mn-NiB SAA成为适合CO 2甲烷化的催化剂。相应地,低的CO 2和CH 4活化势垒使Mn-NiB SAA成为DRM反应的理想选择。突出的是,CH 4的高发光度逐步脱氢结合用于Boudouard反应的低阻隔层可减少表面焦炭的形成。因此,我们认为Mn-NiB SAA可以作为CO 2甲烷化和DRM反应的潜在催化剂。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号