当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Construction of Benzothiophene-Fused N-Heterocycles via Stereotunable Three-Component Domino Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-02-16 , DOI: 10.1021/acs.joc.0c02692 Qingsong Deng 1 , Aimin Yu 1 , Lei Zhang 2 , Xiangtai Meng 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-02-16 , DOI: 10.1021/acs.joc.0c02692 Qingsong Deng 1 , Aimin Yu 1 , Lei Zhang 2 , Xiangtai Meng 1
Affiliation
|
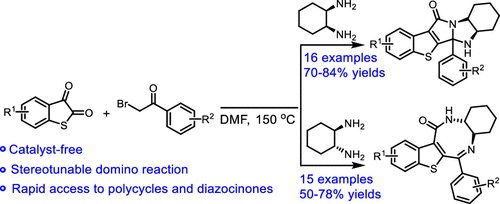
A stereotunable three-component domino strategy among thioisatin, 2-bromo-1-phenylethan-1-one, and cyclohexane-1,2-diamine under catalyst-free conditions was disclosed. A wide range of benzothiophene-fused polycycles and eight-membered N-heterocycles were synthesized by regulating the stereoconfiguration of cyclohexane-1,2-diamines. The detailed mechanism and the origin of the chemoselectivity were explored by density functional calculations. Analysis of the geometrical structures of key transition states revealed that the existence of favorable intramolecular attractions, and the steric effect governed the chemoselectivity observed.
中文翻译:

通过立体可调三组分多米诺反应发散构造苯并噻吩融合的N-杂环
公开了一种在无催化剂条件下的硫代抑素,2-溴-1-苯基乙-1-酮和环己烷-1,2-二胺之间的立体可调三组分多米诺策略。通过调节环己烷-1,2-二胺的立体构型,合成了多种苯并噻吩稠合的多环和八元N-杂环。通过密度泛函计算探索了化学选择性的详细机理和起源。关键过渡态的几何结构分析表明,存在良好的分子内吸引力,并且空间效应支配着所观察到的化学选择性。
更新日期:2021-03-05
中文翻译:

通过立体可调三组分多米诺反应发散构造苯并噻吩融合的N-杂环
公开了一种在无催化剂条件下的硫代抑素,2-溴-1-苯基乙-1-酮和环己烷-1,2-二胺之间的立体可调三组分多米诺策略。通过调节环己烷-1,2-二胺的立体构型,合成了多种苯并噻吩稠合的多环和八元N-杂环。通过密度泛函计算探索了化学选择性的详细机理和起源。关键过渡态的几何结构分析表明,存在良好的分子内吸引力,并且空间效应支配着所观察到的化学选择性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号