当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism and Kinetics of Methane Oxidation to Methanol Catalyzed by AuPd Nanocatalysts at Low Temperature
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-16 , DOI: 10.1021/acscatal.0c04487 Rui Serra-Maia 1 , F. Marc Michel 2 , Temple A. Douglas 3 , Yijin Kang 4 , Eric A. Stach 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-16 , DOI: 10.1021/acscatal.0c04487 Rui Serra-Maia 1 , F. Marc Michel 2 , Temple A. Douglas 3 , Yijin Kang 4 , Eric A. Stach 1
Affiliation
|
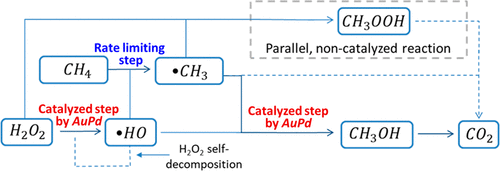
The selective oxidation of methane to methanol at ambient conditions has the potential to enable the use of natural gas obtained in remote areas. We performed multivariate statistical analysis of 137 laboratory experiments to show that gold–palladium nanocatalysts unlock the reaction pathway that forms methanol, but the overall rate of methane oxidation does not correlate with the total number of active sites because the catalyst does not participate in the rate limiting step of the reaction. Using an H2O2 concentration below 2.5 × 10–5 mol/L reduces the H2O2 overuse up to 10,000 times and promotes a methanol selectivity greater than 0.8 without compromising the reaction productivity. These results have enormous practical importance for methane utilization because they explain the poor methane conversion rates obtained thus far and show the path to increase the methanol productivity and reduce the overuse of industrially expensive H2O2.
中文翻译:

AuPd纳米催化剂在低温下甲烷氧化为甲醇的机理和动力学
在环境条件下将甲烷选择性氧化为甲醇具有潜力,可以使用在偏远地区获得的天然气。我们对137个实验室实验进行了多变量统计分析,结果表明金钯纳米催化剂可解锁形成甲醇的反应路径,但甲烷氧化的总速率与活性位点总数无关,因为该催化剂不参与该速率。反应的限制步骤。使用低于2.5×10 –5 mol / L的H 2 O 2浓度会降低H 2 O 2最多可使用10,000次,并在不影响反应生产率的情况下提高甲醇选择性,使其大于0.8。这些结果对于甲烷利用具有极大的实际意义,因为它们解释了迄今为止获得的较差的甲烷转化率,并显示了提高甲醇生产率和减少工业上昂贵的H 2 O 2过度使用的途径。
更新日期:2021-03-05
中文翻译:

AuPd纳米催化剂在低温下甲烷氧化为甲醇的机理和动力学
在环境条件下将甲烷选择性氧化为甲醇具有潜力,可以使用在偏远地区获得的天然气。我们对137个实验室实验进行了多变量统计分析,结果表明金钯纳米催化剂可解锁形成甲醇的反应路径,但甲烷氧化的总速率与活性位点总数无关,因为该催化剂不参与该速率。反应的限制步骤。使用低于2.5×10 –5 mol / L的H 2 O 2浓度会降低H 2 O 2最多可使用10,000次,并在不影响反应生产率的情况下提高甲醇选择性,使其大于0.8。这些结果对于甲烷利用具有极大的实际意义,因为它们解释了迄今为止获得的较差的甲烷转化率,并显示了提高甲醇生产率和减少工业上昂贵的H 2 O 2过度使用的途径。













































 京公网安备 11010802027423号
京公网安备 11010802027423号