当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective and Stereospecific Triflate‐Mediated Intramolecular Schmidt Reaction: Ready Access to Alkaloid Skeletons**
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-02-16 , DOI: 10.1002/anie.202016892
Lars Gnägi 1 , Remo Arnold 1 , Florence Giornal 1 , Harish Jangra 2 , Ajoy Kapat 1 , Erich Nyfeler 1 , Robin M Schärer 1 , Hendrik Zipse 2 , Philippe Renaud 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-02-16 , DOI: 10.1002/anie.202016892
Lars Gnägi 1 , Remo Arnold 1 , Florence Giornal 1 , Harish Jangra 2 , Ajoy Kapat 1 , Erich Nyfeler 1 , Robin M Schärer 1 , Hendrik Zipse 2 , Philippe Renaud 1
Affiliation
|
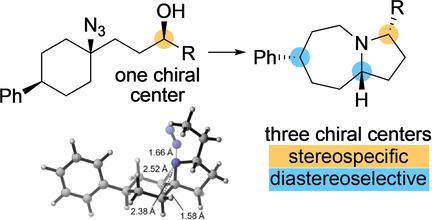
The stereoselectivity and stereospecificity of the triflate‐mediated intramolecular Schmidt reaction of substituted 3‐(1‐azidocyclohexyl)propanol derivatives leading to octahydro‐1H‐pyrrolo[1,2‐a]azepine, the structural skeleton of several important families of alkaloids such as the Stemona alkaloids, has been examined. The reaction involves an initial intramolecular SN2 reaction between the azide moiety and the triflate affording an intermediate spirocyclic aminodiazonoium salt that undergoes the expected 1,2‐shift/N2‐elimination followed by hydride‐mediated iminium salt reduction. Remarkably, chiral alcohols are converted to the azabicyclic derivative with no or limited racemization. The initial asymmetric alcohol center controls the diastereoselectivity of the whole process, leading to the formation of one out of the four possible diastereoisomers of disubstituted octahydro‐1H‐pyrrolo[1,2‐a]azepine. The origin of the stereoselectivity is rationalized based on theoretical calculations. The concise synthesis of (−)‐(cis)‐3‐propylindolizidine and (−)‐(cis)‐3‐butyllehmizidine, two alkaloids found in the venom of workers of the ant Myrmicaria melanogaster, is reported.
中文翻译:

立体选择性和立体特异性三氟甲磺酸介导的分子内施密特反应:易于接触生物碱骨架**
三氟甲磺酸酯介导的3-(1-叠氮基环己基)丙醇衍生物的三氟甲磺酸酯介导的分子内Schmidt反应的立体选择性和立体选择性,导致八氢-1H-吡咯并[1,2-a]氮杂,如几个重要生物碱家族的结构骨架Stemona生物碱已经过检查。该反应涉及叠氮化物部分与三氟甲磺酸酯之间的分子内初始S N 2反应,从而提供中间的螺环氨基重氮盐,该盐经历了预期的1,2-shift / N 2消除,然后氢化物介导的亚胺盐还原。显着地,手性醇在没有外消旋或外消旋作用有限的情况下被转化为氮杂双环衍生物。最初的不对称醇中心控制着整个过程的非对映选择性,从而导致双取代八氢-1H-吡咯并[1,2-a]氮杂烷的四种可能的非对映异构体中的一种形成。立体选择性的起源是根据理论计算合理化的。据报道,在蚂蚁黑粉菌工人的毒液中发现了两种生物碱,即(-)-(顺式)-3-丙基吲哚唑烷和(-)-(顺式)-3-丁基吲哚并咪唑的简明合成。
更新日期:2021-04-23
中文翻译:

立体选择性和立体特异性三氟甲磺酸介导的分子内施密特反应:易于接触生物碱骨架**
三氟甲磺酸酯介导的3-(1-叠氮基环己基)丙醇衍生物的三氟甲磺酸酯介导的分子内Schmidt反应的立体选择性和立体选择性,导致八氢-1H-吡咯并[1,2-a]氮杂,如几个重要生物碱家族的结构骨架Stemona生物碱已经过检查。该反应涉及叠氮化物部分与三氟甲磺酸酯之间的分子内初始S N 2反应,从而提供中间的螺环氨基重氮盐,该盐经历了预期的1,2-shift / N 2消除,然后氢化物介导的亚胺盐还原。显着地,手性醇在没有外消旋或外消旋作用有限的情况下被转化为氮杂双环衍生物。最初的不对称醇中心控制着整个过程的非对映选择性,从而导致双取代八氢-1H-吡咯并[1,2-a]氮杂烷的四种可能的非对映异构体中的一种形成。立体选择性的起源是根据理论计算合理化的。据报道,在蚂蚁黑粉菌工人的毒液中发现了两种生物碱,即(-)-(顺式)-3-丙基吲哚唑烷和(-)-(顺式)-3-丁基吲哚并咪唑的简明合成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号