当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Activation of Atomic Layer-Deposited Cobalt Phosphate Electrocatalysts for Water Oxidation
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-15 , DOI: 10.1021/acscatal.0c04933 Ruoyu Zhang 1, 2 , Gerben van Straaten 1 , Valerio di Palma 1 , Georgios Zafeiropoulos 2 , Mauritius C M van de Sanden 1, 2 , Wilhelmus M M Kessels 1 , Mihalis N Tsampas 2 , Mariadriana Creatore 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-15 , DOI: 10.1021/acscatal.0c04933 Ruoyu Zhang 1, 2 , Gerben van Straaten 1 , Valerio di Palma 1 , Georgios Zafeiropoulos 2 , Mauritius C M van de Sanden 1, 2 , Wilhelmus M M Kessels 1 , Mihalis N Tsampas 2 , Mariadriana Creatore 1
Affiliation
|
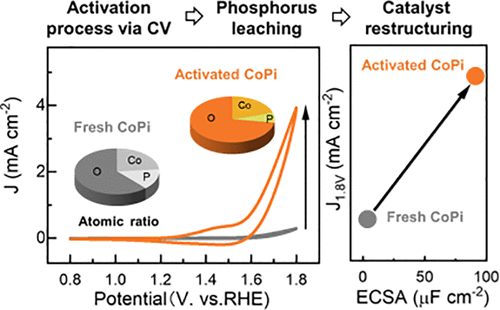
The development of efficient and stable earth-abundant water oxidation catalysts is vital for economically feasible water-splitting systems. Cobalt phosphate (CoPi)-based catalysts belong to the relevant class of nonprecious electrocatalysts studied for the oxygen evolution reaction (OER). In this work, an in-depth investigation of the electrochemical activation of CoPi-based electrocatalysts by cyclic voltammetry (CV) is presented. Atomic layer deposition (ALD) is adopted because it enables the synthesis of CoPi films with cobalt-to-phosphorous ratios between 1.4 and 1.9. It is shown that the pristine chemical composition of the CoPi film strongly influences its OER activity in the early stages of the activation process as well as after prolonged exposure to the electrolyte. The best performing CoPi catalyst, displaying a current density of 3.9 mA cm–2 at 1.8 V versus reversible hydrogen electrode and a Tafel slope of 155 mV/dec at pH 8.0, is selected for an in-depth study of the evolution of its electrochemical properties, chemical composition, and electrochemical active surface area (ECSA) during the activation process. Upon the increase of the number of CV cycles, the OER performance increases, in parallel with the development of a noncatalytic wave in the CV scan, which points out to the reversible oxidation of Co2+ species to Co3+ species. X-ray photoelectron spectroscopy and Rutherford backscattering measurements indicate that phosphorous progressively leaches out the CoPi film bulk upon prolonged exposure to the electrolyte. In parallel, the ECSA of the films increases by up to a factor of 40, depending on the initial stoichiometry. The ECSA of the activated CoPi films shows a universal linear correlation with the OER activity for the whole range of CoPi chemical composition. It can be concluded that the adoption of ALD in CoPi-based electrocatalysis enables, next to the well-established control over film growth and properties, to disclose the mechanisms behind the CoPi electrocatalyst activation.
中文翻译:

原子层沉积磷酸钴电催化剂的电化学活化用于水氧化
开发高效稳定的地球丰富的水氧化催化剂对于经济可行的水分解系统至关重要。磷酸钴 (CoPi) 基催化剂属于用于析氧反应 (OER) 研究的相关非贵金属电催化剂类别。在这项工作中,通过循环伏安法(CV)对 CoPi 基电催化剂的电化学活化进行了深入研究。采用原子层沉积(ALD)是因为它可以合成钴磷比在1.4至1.9之间的CoPi薄膜。结果表明,CoPi 薄膜的原始化学成分在活化过程的早期阶段以及长时间暴露于电解质后强烈影响其 OER 活性。性能最佳的 CoPi 催化剂在 1.8 V 电压下相对于可逆氢电极显示出 3.9 mA cm –2的电流密度,在 pH 8.0 下的塔菲尔斜率为 155 mV/dec,被选中用于深入研究其电化学演化活化过程中的性能、化学成分和电化学活性表面积(ECSA)。随着CV循环次数的增加,OER性能提高,同时CV扫描中出现非催化波,这表明Co 2+物质可逆氧化为Co 3+物质。 X 射线光电子能谱和卢瑟福背向散射测量表明,在长时间暴露于电解质时,磷会逐渐从 CoPi 薄膜本体中浸出。与此同时,薄膜的 ECSA 增加了 40 倍,具体取决于初始化学计量。 对于整个 CoPi 化学成分范围,活化 CoPi 薄膜的 ECSA 与 OER 活性呈现普遍的线性相关性。可以得出的结论是,在基于 CoPi 的电催化中采用 ALD,除了对薄膜生长和性能进行完善的控制之外,还可以揭示 CoPi 电催化剂激活背后的机制。
更新日期:2021-03-05
中文翻译:

原子层沉积磷酸钴电催化剂的电化学活化用于水氧化
开发高效稳定的地球丰富的水氧化催化剂对于经济可行的水分解系统至关重要。磷酸钴 (CoPi) 基催化剂属于用于析氧反应 (OER) 研究的相关非贵金属电催化剂类别。在这项工作中,通过循环伏安法(CV)对 CoPi 基电催化剂的电化学活化进行了深入研究。采用原子层沉积(ALD)是因为它可以合成钴磷比在1.4至1.9之间的CoPi薄膜。结果表明,CoPi 薄膜的原始化学成分在活化过程的早期阶段以及长时间暴露于电解质后强烈影响其 OER 活性。性能最佳的 CoPi 催化剂在 1.8 V 电压下相对于可逆氢电极显示出 3.9 mA cm –2的电流密度,在 pH 8.0 下的塔菲尔斜率为 155 mV/dec,被选中用于深入研究其电化学演化活化过程中的性能、化学成分和电化学活性表面积(ECSA)。随着CV循环次数的增加,OER性能提高,同时CV扫描中出现非催化波,这表明Co 2+物质可逆氧化为Co 3+物质。 X 射线光电子能谱和卢瑟福背向散射测量表明,在长时间暴露于电解质时,磷会逐渐从 CoPi 薄膜本体中浸出。与此同时,薄膜的 ECSA 增加了 40 倍,具体取决于初始化学计量。 对于整个 CoPi 化学成分范围,活化 CoPi 薄膜的 ECSA 与 OER 活性呈现普遍的线性相关性。可以得出的结论是,在基于 CoPi 的电催化中采用 ALD,除了对薄膜生长和性能进行完善的控制之外,还可以揭示 CoPi 电催化剂激活背后的机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号