当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphine-Free Manganese Catalyst Enables Selective Transfer Hydrogenation of Nitriles to Primary and Secondary Amines Using Ammonia–Borane
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-15 , DOI: 10.1021/acscatal.0c05406 Koushik Sarkar 1 , Kuhali Das 1 , Abhishek Kundu 2 , Debashis Adhikari 2 , Biplab Maji 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-15 , DOI: 10.1021/acscatal.0c05406 Koushik Sarkar 1 , Kuhali Das 1 , Abhishek Kundu 2 , Debashis Adhikari 2 , Biplab Maji 1
Affiliation
|
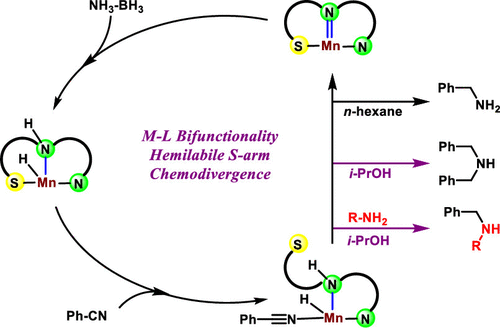
Herein we report the synthesis of primary and secondary amines by nitrile hydrogenation, employing a borrowing hydrogenation strategy. A class of phosphine-free manganese(I) complexes bearing sulfur side arms catalyzed the reaction under mild reaction conditions, where ammonia–borane is used as the source of hydrogen. The synthetic protocol is chemodivergent, as the final product is either primary or secondary amine, which can be controlled by changing the catalyst structure and the polarity of the reaction medium. The significant advantage of this method is that the protocol operates without externally added base or other additives as well as obviates the use of high-pressure dihydrogen gas required for other nitrile hydrogenation reactions. Utilizing this method, a wide variety of primary and symmetric and asymmetric secondary amines were synthesized in high yields. A mechanistic study involving kinetic experiments and high-level DFT computations revealed that both outer-sphere dehydrogenation and inner-sphere hydrogenation were predominantly operative in the catalytic cycle.
中文翻译:

无磷锰催化剂可使用氨-硼烷将腈选择性转移加氢成伯胺和仲胺
在本文中,我们报告了采用借位氢化策略通过腈加氢合成伯胺和仲胺的方法。一类带有硫侧臂的无膦锰(I)络合物在温和的反应条件下催化反应,其中氨硼烷用作氢源。合成规程是化学发散的,因为最终产物是伯胺或仲胺,可以通过改变催化剂结构和反应介质的极性来控制。该方法的显着优点是该方案无需外部添加碱或其他添加剂即可运行,并且避免了其他腈加氢反应所需的高压二氢气体的使用。利用这种方法 以高收率合成了各种各样的伯胺,对称胺和不对称仲胺。涉及动力学实验和高水平DFT计算的机理研究表明,外球面脱氢和内球面加氢在催化循环中均主要起作用。
更新日期:2021-03-05
中文翻译:

无磷锰催化剂可使用氨-硼烷将腈选择性转移加氢成伯胺和仲胺
在本文中,我们报告了采用借位氢化策略通过腈加氢合成伯胺和仲胺的方法。一类带有硫侧臂的无膦锰(I)络合物在温和的反应条件下催化反应,其中氨硼烷用作氢源。合成规程是化学发散的,因为最终产物是伯胺或仲胺,可以通过改变催化剂结构和反应介质的极性来控制。该方法的显着优点是该方案无需外部添加碱或其他添加剂即可运行,并且避免了其他腈加氢反应所需的高压二氢气体的使用。利用这种方法 以高收率合成了各种各样的伯胺,对称胺和不对称仲胺。涉及动力学实验和高水平DFT计算的机理研究表明,外球面脱氢和内球面加氢在催化循环中均主要起作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号