当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of the Solubility of 4,4′-Oxydianiline in Four Binary Solvent Mixtures from T = 293.15 to 333.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-02-14 , DOI: 10.1021/acs.jced.0c00965
Shaopo Wang , Lihong Jia , Qiuxiang Yin , Baohong Hou , Ling Zhou , Degui Wu , Ketao Shi , Xinyuan Zhang
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-02-14 , DOI: 10.1021/acs.jced.0c00965
Shaopo Wang , Lihong Jia , Qiuxiang Yin , Baohong Hou , Ling Zhou , Degui Wu , Ketao Shi , Xinyuan Zhang
|
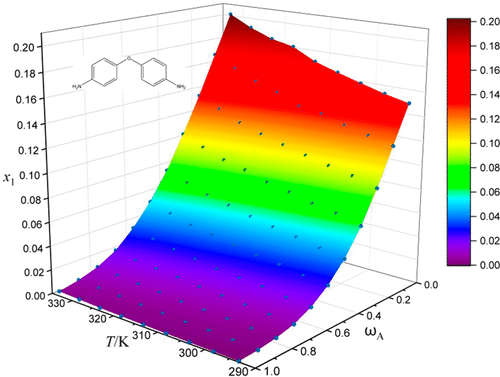
4,4′-Oxydianiline (ODA) is a versatile fine chemical intermediate with huge market demand. The purification of ODA is accomplished by crystallization with some high-boiling-point liquids as solvents, but the product yields are usually low in these pure solvents. Using a mixture of a poor solvent such as ethanol and a high-boiling-point liquid as the solvent can improve the yield of ODA, and the design of the new crystallization process requires the solubility properties of ODA in the new solvent system. However, these data have never been reported. In this work, the solubility of 4,4′-oxidianiline in dimethyl sulfoxide + ethanol, N,N-dimethylacetamide + ethanol, N,N-dimethylformamide + ethanol, and ethylene glycol monomethyl ether + ethanol binary solvent mixtures from 293.15 to 333.15 K was measured by the gravimetric method under atmospheric pressure. The solubility of ODA increases with the increase in temperature and decreases with the increase in the mass fraction of ethanol. The experimental solubility data of ODA were correlated by the Van’t Hoff equation, the modified Apelblat equation, the CNIBS/R–K model, the Van’t Hoff–Jouyban–Acree model, and the Apelblat–Jouyban–Acree model. The calculated values are in good agreement with the measured solubility data for all five models, among which the modified Apelblat equation provides the best correlation.
中文翻译:

在T = 293.15至333.15 K的四种二元溶剂混合物中4,4'-氧代二苯胺溶解度的测量和相关性
4,4'-氧化二苯胺(ODA)是一种多功能的精细化工中间体,具有巨大的市场需求。ODA的纯化是通过使用一些高沸点液体作为溶剂进行结晶来完成的,但是在这些纯溶剂中,产品的收率通常较低。使用不良溶剂(例如乙醇)和高沸点液体的混合物作为溶剂可以提高ODA的产率,新结晶工艺的设计要求ODA在新溶剂系统中具有溶解性。但是,这些数据从未被报道过。在这项工作中,4,4'-氧苯胺在二甲亚砜+乙醇,N,N-N-二甲基乙酰胺+乙醇,N,N中的溶解度通过重量法在大气压下测量293.15至333.15 K的-二甲基甲酰胺+乙醇和乙二醇单甲醚+乙醇二元溶剂混合物。ODA的溶解度随着温度的升高而增加,并且随着乙醇的质量分数的增加而降低。ODA的实验溶解度数据通过Van't Hoff方程,修正的Apelblat方程,CNIBS / R–K模型,Van't Hoff–Jouyban–Acree模型和Apelblat–Jouyban–Acree模型进行关联。所有五个模型的计算值与测得的溶解度数据高度吻合,其中改进的Apelblat方程提供了最佳相关性。
更新日期:2021-03-11
中文翻译:

在T = 293.15至333.15 K的四种二元溶剂混合物中4,4'-氧代二苯胺溶解度的测量和相关性
4,4'-氧化二苯胺(ODA)是一种多功能的精细化工中间体,具有巨大的市场需求。ODA的纯化是通过使用一些高沸点液体作为溶剂进行结晶来完成的,但是在这些纯溶剂中,产品的收率通常较低。使用不良溶剂(例如乙醇)和高沸点液体的混合物作为溶剂可以提高ODA的产率,新结晶工艺的设计要求ODA在新溶剂系统中具有溶解性。但是,这些数据从未被报道过。在这项工作中,4,4'-氧苯胺在二甲亚砜+乙醇,N,N-N-二甲基乙酰胺+乙醇,N,N中的溶解度通过重量法在大气压下测量293.15至333.15 K的-二甲基甲酰胺+乙醇和乙二醇单甲醚+乙醇二元溶剂混合物。ODA的溶解度随着温度的升高而增加,并且随着乙醇的质量分数的增加而降低。ODA的实验溶解度数据通过Van't Hoff方程,修正的Apelblat方程,CNIBS / R–K模型,Van't Hoff–Jouyban–Acree模型和Apelblat–Jouyban–Acree模型进行关联。所有五个模型的计算值与测得的溶解度数据高度吻合,其中改进的Apelblat方程提供了最佳相关性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号