Tetrahedron ( IF 2.1 ) Pub Date : 2021-02-15 , DOI: 10.1016/j.tet.2021.131981 Yang Kang , Yun He , Jingzhi Sui , Tao Wang , Yong Liang , Zunting Zhang
|
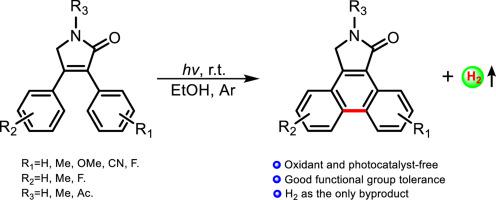
An efficient, oxidant and photocatalyst-free approach for the synthesis of polycyclic-fused isoindolinone derivatives is reported via annulation of 3,4-diphenyl-3-pyrrolin-2-ones along with release of the hydrogen gas under an argon atmosphere in EtOH by irradiation with a 500 W mercury lamp at room temperature. The described approach is atom-economic and environmentally friendly and tolerates various electron-donating and electron-withdrawing groups. In addition, selected annulation products, dibenzo[e,g]isoindole-1-ones, were successfully oxidized into dibenzo[e,g]isoindole-1,3(2H)- diones in DMSO in the presence of CH3ONa at room temperature.
中文翻译:

3,4-二苯基-1 H-吡咯-2(5 H)-ones的光诱导分子内环化反应合成二苯并[ e,g ] isoindol-1-ones
通过3,4-二苯基-3-吡咯啉-2-酮的环化反应,以及在氩气中在氩气氛下通过氢气释放氢气,报道了一种高效,无氧化剂,无光催化剂的合成多环稠合异吲哚啉酮衍生物的方法。在室温下用500 W汞灯照射。所描述的方法是原子经济的和环境友好的,并且可以耐受各种给电子和吸电子基团。另外,在CH 3 ONa存在下,于DMSO中,将选定的环化产物二苯并[ e,g ]异吲哚-1-酮成功氧化为二苯并[ e,g ]异吲哚-1,3(2 H)-二酮。室内温度。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号