Journal of Chromatography B ( IF 2.8 ) Pub Date : 2021-02-14 , DOI: 10.1016/j.jchromb.2021.122582 Peng Li , Xiao-Hui Ma , Ling Jin , Juan Chen
|
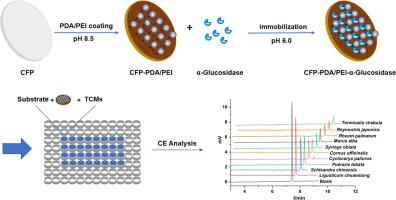
In this work, cellulose filter paper (CFP), which is inexpensive and commercially available, was used as the carrier, and the immobilized α-glucosidase was obtained by two steps: firstly, the surface of CFP was modified by polydopamine/polyethyleneimine (PDA/PEI) co-deposition method to obtain CFP-PDA/PEI with a uniform coating of rich positive charge; subsequently, α-glucosidase was immobilized on the CFP-PDA/PEI by electrostatic adsorption. The free enzyme and immobilized enzyme have the same optimal temperature (70℃) and pH (8.0), and their Km is similar, which is 2.2 and 2.8, respectively. These results show that the immobilization process does not change the properties of the enzyme greatly. The immobilized enzyme still maintains 75.6% of its initial activity after 10 repeated uses, showing good reusability. The excellent repeatability (RSD = 2.2%, n = 5) and the verification of competitive inhibitor (acarbose) illustrates the reliability of the immobilized enzymes for enzyme inhibitor screening. Finally, combined with CE, a screening method based immobilized α-glucosidase was proposed and applied to screen the α-glucosidase inhibitory from 10 kinds of Traditional Chinese medicines (TCMs) in vitro. The results indicated that the method was a very effective tool for screening potential α-glucosidase inhibitors from TCMs.































 京公网安备 11010802027423号
京公网安备 11010802027423号