Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-02-15 , DOI: 10.1016/j.cej.2021.128967 Nighat Zarshad , Anis Ur Rahman , Jianghua Wu , Asad Ali , Fazal Raziq , Lu Han , Peng Wang , Guigen Li , Henmei Ni
|
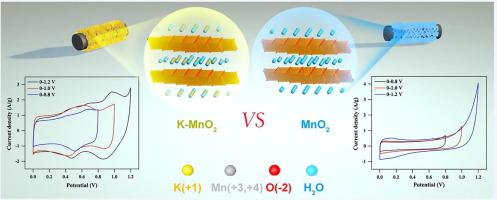
Based on the redox couple reaction of Mn3+/Mn4+, the theoretical capacitance of manganese dioxide (MnO2) has a very high value of 1370 F/g but the specific capacitance of MnO2 in the reported literature is still under 300 F/g. The potential window of MnO2 in the previously published studies is approximately 1 V which, considering E = ½CV2, limits the power and energy densities far below the theoretical value. In this work, we developed an ultra-thin K+ doped δ-MnO2 nanosheets array electrode the potential window of which extended to 0-1.2 V with a highly reversible capacitance of 366 F/g. When the potential window reaches 1.2 V, the fast-redox reaction of MnO2 and the K+ intercalation/deintercalation process restrains water decomposition in kinetics. A high potency aqueous asymmetric supercapacitor (K-MnO2//AC) was drafted with K-MnO2 as a positive electrode which exhibited a stable working potential window of 0-2.2 V in 1 M Na2SO4 aqueous electrolyte. This device has demonstrated an excellent energy density of 56 Wh/kg at a power density of 550 W/kg and an ultralong cycle performance with capacitance retention of 98% over 10000 cycles at the current density of 10 A/g. This approach leads to new prospects for emerging high workable potential window aqueous energy storage devices.
中文翻译:

掺K的MnO 2 @碳布超级电容器具有增强的能量密度和宽电位窗口
基于Mn 3+ / Mn 4+的氧化还原偶联反应,二氧化锰(MnO 2)的理论电容值非常高,为1370 F / g,但所报道的文献中MnO 2的比电容仍在300以下氟/克 的MnO的电位窗口2在先前公布的研究是约1 V其中,考虑到E =½CV 2,限制远低于理论值的功率和能量密度。在这项工作中,我们开发了一种超薄ķ + δ掺杂的MnO 2纳米片阵列电极,其电位窗口扩展至0-1.2 V,具有366 F / g的高度可逆电容。当电势窗口达到1.2 V时,MnO 2的快速氧化还原反应和K +嵌入/去嵌入过程抑制了水的动力学分解。以K-MnO 2为正极绘制了高效的水不对称超级电容器(K-MnO 2 // AC),该溶液在1 M Na 2 SO 4中显示0-2.2 V的稳定工作电位窗口水性电解质。该器件在550 W / kg的功率密度下具有出色的56 Wh / kg的能量密度,在10 A / g的电流密度下,在10000次循环中具有98%的电容保持率,超长循环性能。这种方法为新兴的高可行的潜在窗口含水储能设备带来了新的前景。































 京公网安备 11010802027423号
京公网安备 11010802027423号