Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-02-14 , DOI: 10.1016/j.cej.2021.128923 Chen Wang , Chao Xiong , Yali He , Chen Yang , Xiteng Li , Jianzhong Zheng , Shixing Wang
|
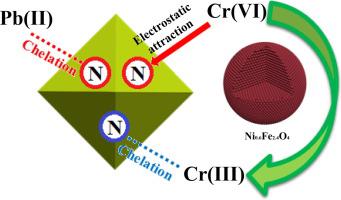
A novel magnetic Zr-MOF named Ni0.6Fe2.4O4-UiO-66-PEI was synthesized by modifying UiO-66-NH2 with Ni0.6Fe2.4O4 and polyethyleneimine and its sorption characteristics for two representing heavy metals Pb(II) and Cr(VI) in water were examined. This material showed good thermal stability and was sufficiently magnetic to allow effective solid-liquid separation. The material has a maximum adsorption capacity of 273.2 mg/g for Pb(II) at pH 4.0 and 428.6 mg/g for Cr(VI) at pH 3.0, as obtained from the model fitting by the Hill equation. The sorption kinetics for both heavy metals on the material was fast, with adsorption equilibrium established in 60 min and 30 min for Pb(II) and Cr(VI), respectively. Model fitting of the kinetic data showed that Pb(II) and Cr(VI) sorption on Ni0.6Fe2.4O4-UiO-66-PEI followed the pseudo-second-order model. This material had good selectivity for competing ions, and could maintain in good binding capacities after 5 times of regeneration. Adsorption mechanisms for Pb(II) and Cr(VI) by Ni0.6Fe2.4O4-UiO-66-PEI were proposed by analyzing FTIR, XPS and the electrokinetic property of the material. Specifically, lead adsorption on Ni0.6Fe2.4O4-UiO-66-PEI could be attributed to chelation of Pb(II) with the imine/amine functional groups in the material’s matrix, while Cr(VI) adsorption might stem from a combination of electrostatic attraction, chelation and redox reaction. It seems that Ni0.6Fe2.4O4-UiO-66-PEI could be used as a green and environmental-friendly material for heavy metals removal, particularly from acidic water streams.
中文翻译:

磁性Zr-MOF的简便制备以吸附水中的Pb(II)和Cr(VI):吸附特性和机理
名为镍的新型磁性Zr的MOF 0.6铁2.4 ø 4通过修饰UIO-66-NH合成-UiO-66-PEI 2用Ni 0.6铁2.4 Ò 4研究了聚乙烯亚胺及其在水中两种代表重金属的Pb(II)和Cr(VI)的吸附特性。该材料显示出良好的热稳定性,并且具有足够的磁性以允许有效的固液分离。通过希尔方程拟合得到的材料在pH 4.0下对Pb(II)的最大吸附容量为273.2 mg / g,在pH 3.0下对Cr(VI)的最大吸附容量为428.6 mg / g。两种重金属在材料上的吸附动力学都很快,Pb(II)和Cr(VI)分别在60分钟和30分钟内建立了吸附平衡。动力学数据的模型拟合表明,Pb(II)和Cr(VI)在Ni 0.6 Fe 2.4 O 4上的吸附-UiO-66-PEI遵循伪二级模型。该材料对竞争离子具有良好的选择性,并且在5次再生后可以保持良好的结合能力。通过分析FTIR,XPS和材料的电动特性,提出了Ni 0.6 Fe 2.4 O 4 -UiO-66-PEI对Pb(II)和Cr(VI)的吸附机理。具体而言,Ni 0.6 Fe 2.4 O 4 -UiO-66-PEI上的铅吸附可能归因于Pb(II)与材料基质中的亚胺/胺官能团的螯合,而Cr(VI)吸附可能源于两者的结合。静电吸引,螯合和氧化还原反应。看来Ni 0.6 Fe 2.4O 4 -UiO-66-PEI可以用作绿色环保材料,特别是从酸性水流中去除重金属。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号