当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconstitution of a Highly Reducing Type II PKS System Reveals 6π-Electrocyclization Is Required for o-Dialkylbenzene Biosynthesis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-12 , DOI: 10.1021/jacs.0c13378
Jie Zhang 1 , Satoshi Yuzawa 1 , Wei Li Thong 1 , Tetsuro Shinada 2 , Makoto Nishiyama 3, 4 , Tomohisa Kuzuyama 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-12 , DOI: 10.1021/jacs.0c13378
Jie Zhang 1 , Satoshi Yuzawa 1 , Wei Li Thong 1 , Tetsuro Shinada 2 , Makoto Nishiyama 3, 4 , Tomohisa Kuzuyama 1, 4
Affiliation
|
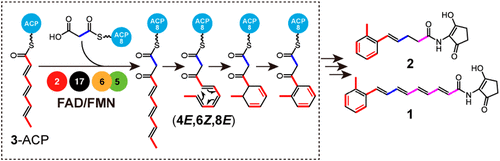
Natural products containing an o-dialkylbenzene moiety exhibit a wide variety of bioactivities, including antibacterial, antifungal, antitumor, and antiangiogenic activities. However, the biosynthetic scheme of the o-dialkylbenzene moiety remains unclear. In this study, we identified the biosynthetic gene cluster (BGC) of compounds 1 and 2 in Streptomyces sp. SANK 60404, which contains a rare o-dialkylbenzene moiety, and successfully reconstituted the biosynthesis of 1 using 22 recombinant enzymes in vitro. Our study established a biosynthetic route for the o-tolyl group within the o-dialkylbenzene moiety, where the triene intermediate 3 loaded onto a unique acyl carrier protein (ACP) is elongated by a specific ketosynthase–chain length factor pair of a type II polyketide synthase system with the aid of a putative isomerase to be termed “electrocyclase” and a thioesterase-like enzyme in the BGC. The C2-elongated all-trans diketo–triene intermediate is subsequently isomerized to the 6Z configuration by the electrocyclase to allow intramolecular 6π-electrocyclization, followed by coenzyme FAD/FMN-dependent dehydrogenation. Bioinformatics analysis showed that the key genes are all conserved in BGCs of natural products containing an o-dialkylbenzene moiety, suggesting that the proposed biosynthetic scheme is a common strategy to form o-dialkylbenzenes in nature.
中文翻译:

高度还原 II 型 PKS 系统的重建表明邻二烷基苯生物合成需要 6π-电环化
含有邻二烷基苯部分的天然产物表现出多种生物活性,包括抗菌、抗真菌、抗肿瘤和抗血管生成活性。然而,邻二烷基苯部分的生物合成方案仍不清楚。在这项研究中,我们鉴定的化合物的生物合成基因簇(BGC)1和2在链霉菌属。SANK 60404 含有稀有的邻二烷基苯部分,并在体外使用 22 种重组酶成功重建了1的生物合成。我们的研究建立了一个生物合成途径Ø的内甲苯基组Ø-二烷基苯部分,其中加载到独特酰基载体蛋白 (ACP) 上的三烯中间体3被 II 型聚酮化合物合酶系统的特定酮合酶-链长因子对在被称为“电环化酶”的推定异构酶的帮助下延长和 BGC 中的硫酯酶样酶。C 2延伸的全反式二酮三烯中间体随后通过电环化酶异构化为 6 Z构型,以允许分子内 6π 电环化,然后进行辅酶 FAD/FMN 依赖性脱氢。生物信息学分析表明,关键基因在含有o的天然产物的 BGC 中均保守-二烷基苯部分,表明所提出的生物合成方案是在自然界中形成邻二烷基苯的常见策略。
更新日期:2021-02-24
中文翻译:

高度还原 II 型 PKS 系统的重建表明邻二烷基苯生物合成需要 6π-电环化
含有邻二烷基苯部分的天然产物表现出多种生物活性,包括抗菌、抗真菌、抗肿瘤和抗血管生成活性。然而,邻二烷基苯部分的生物合成方案仍不清楚。在这项研究中,我们鉴定的化合物的生物合成基因簇(BGC)1和2在链霉菌属。SANK 60404 含有稀有的邻二烷基苯部分,并在体外使用 22 种重组酶成功重建了1的生物合成。我们的研究建立了一个生物合成途径Ø的内甲苯基组Ø-二烷基苯部分,其中加载到独特酰基载体蛋白 (ACP) 上的三烯中间体3被 II 型聚酮化合物合酶系统的特定酮合酶-链长因子对在被称为“电环化酶”的推定异构酶的帮助下延长和 BGC 中的硫酯酶样酶。C 2延伸的全反式二酮三烯中间体随后通过电环化酶异构化为 6 Z构型,以允许分子内 6π 电环化,然后进行辅酶 FAD/FMN 依赖性脱氢。生物信息学分析表明,关键基因在含有o的天然产物的 BGC 中均保守-二烷基苯部分,表明所提出的生物合成方案是在自然界中形成邻二烷基苯的常见策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号