当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of 5-(Trifluoromethyl)-1,2,4-oxadiazole-Based Class IIa HDAC Inhibitors for Huntington’s Disease
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2021-02-11 , DOI: 10.1021/acsmedchemlett.0c00532 Andrew J Stott 1 , Michel C Maillard 2 , Vahri Beaumont 2 , David Allcock 1 , Omar Aziz 1 , Alexander H Borchers 2 , Wesley Blackaby 1 , Perla Breccia 1 , Gillian Creighton-Gutteridge 1 , Alan F Haughan 1 , Rebecca E Jarvis 1 , Christopher A Luckhurst 1 , Kim L Matthews 1 , George McAllister 1 , Scott Pollack 1 , Elizabeth Saville-Stones 1 , Amanda J Van de Poël 1 , Huw D Vater 1 , Julie Vann 1 , Rachel Williams 1 , Dawn Yates 1 , Ignacio Muñoz-Sanjuán 2 , Celia Dominguez 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2021-02-11 , DOI: 10.1021/acsmedchemlett.0c00532 Andrew J Stott 1 , Michel C Maillard 2 , Vahri Beaumont 2 , David Allcock 1 , Omar Aziz 1 , Alexander H Borchers 2 , Wesley Blackaby 1 , Perla Breccia 1 , Gillian Creighton-Gutteridge 1 , Alan F Haughan 1 , Rebecca E Jarvis 1 , Christopher A Luckhurst 1 , Kim L Matthews 1 , George McAllister 1 , Scott Pollack 1 , Elizabeth Saville-Stones 1 , Amanda J Van de Poël 1 , Huw D Vater 1 , Julie Vann 1 , Rachel Williams 1 , Dawn Yates 1 , Ignacio Muñoz-Sanjuán 2 , Celia Dominguez 2
Affiliation
|
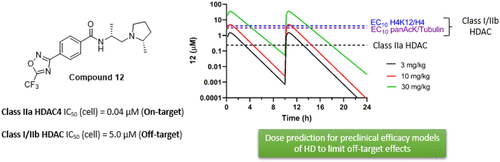
Using an iterative structure–activity relationship driven approach, we identified a CNS-penetrant 5-(trifluoromethyl)-1,2,4-oxadiazole (TFMO, 12) with a pharmacokinetic profile suitable for probing class IIa histone deacetylase (HDAC) inhibition in vivo. Given the lack of understanding of endogenous class IIa HDAC substrates, we developed a surrogate readout to measure compound effects in vivo, by exploiting the >100-fold selectivity compound 12 exhibits over class I/IIb HDACs. We achieved adequate brain exposure with compound 12 in mice to estimate a class I/IIb deacetylation EC50, using class I substrate H4K12 acetylation and global acetylation levels as a pharmacodynamic readout. We observed excellent correlation between the compound 12 in vivo pharmacodynamic response and in vitro class I/IIb cellular activity. Applying the same relationship to class IIa HDAC inhibition, we estimated the compound 12 dose required to inhibit class IIa HDAC activity, for use in preclinical models of Huntington’s disease.
中文翻译:

5-(三氟甲基)-1,2,4-恶二唑基 IIa 类 HDAC 抑制剂对亨廷顿病的评价
使用迭代的结构-活性关系驱动方法,我们鉴定了一种 CNS 渗透剂 5-(三氟甲基)-1,2,4-恶二唑 (TFMO, 12 ),其药代动力学特征适用于探测 IIa 类组蛋白脱乙酰酶 (HDAC) 抑制体内。鉴于缺乏对内源性 IIa 类 HDAC 底物的了解,我们开发了一种替代读数来测量体内化合物的作用,通过利用比 I/IIb 类 HDAC 展示的 > 100 倍的选择性化合物12 。我们在小鼠中用化合物12实现了足够的脑暴露,以估计 I/IIb 类脱乙酰化 EC 50,使用 I 类底物 H4K12 乙酰化和整体乙酰化水平作为药效学读数。我们观察到化合物12的体内药效学反应和体外 I/IIb 类细胞活性之间的极好相关性。将相同的关系应用于 IIa 类 HDAC 抑制,我们估计了抑制 IIa 类 HDAC 活性所需的化合物12剂量,用于亨廷顿病的临床前模型。
更新日期:2021-03-11
中文翻译:

5-(三氟甲基)-1,2,4-恶二唑基 IIa 类 HDAC 抑制剂对亨廷顿病的评价
使用迭代的结构-活性关系驱动方法,我们鉴定了一种 CNS 渗透剂 5-(三氟甲基)-1,2,4-恶二唑 (TFMO, 12 ),其药代动力学特征适用于探测 IIa 类组蛋白脱乙酰酶 (HDAC) 抑制体内。鉴于缺乏对内源性 IIa 类 HDAC 底物的了解,我们开发了一种替代读数来测量体内化合物的作用,通过利用比 I/IIb 类 HDAC 展示的 > 100 倍的选择性化合物12 。我们在小鼠中用化合物12实现了足够的脑暴露,以估计 I/IIb 类脱乙酰化 EC 50,使用 I 类底物 H4K12 乙酰化和整体乙酰化水平作为药效学读数。我们观察到化合物12的体内药效学反应和体外 I/IIb 类细胞活性之间的极好相关性。将相同的关系应用于 IIa 类 HDAC 抑制,我们估计了抑制 IIa 类 HDAC 活性所需的化合物12剂量,用于亨廷顿病的临床前模型。































 京公网安备 11010802027423号
京公网安备 11010802027423号