Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2021-02-12 , DOI: 10.1016/j.jallcom.2021.159174 Xiyan Liu , Yu Gong
|
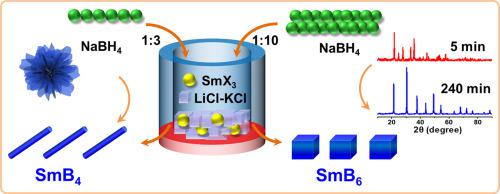
Samarium borides exhibit unique electrical and magnetic properties that have potential applications in a number of fields. However, it is still challenging to obtain crystalline samarium borides with controllable stoichiometry and morphology by simple synthetic methods. Herein, we report the one-pot synthesis of two samarium boride phases via the reactions between samarium halides and NaBH4 in molten LiCl-KCl. The stoichiometry and morphology were controlled by varying the precursor ratio and reaction time. The crystalline SmB4 possesses rod-shaped structure with diameters around 60–90 nm, and nanocubes around 50–100 nm were obtained for SmB6. On the basis of XRD and SEM results, the SmB4 nanorods obtained at a Sm/B ratio of 1:3 evolve from the initially formed SmB4 nanofilms, while formation of the SmB6 nanocubes at a Sm/B ratio of 1:10 is mediated by SmB4. The transition from film-shaped SmB4 to cubic SmB6 most likely results from the destruction of the SmB4 nanocrystal upon partial reduction of Sm3+ to Sm2+ in the presence of excess NaBH4, which is consistent with the change in oxidation state of samarium from XANES analysis. The SmB4 nanorods and SmB6 nanocubes synthesized in molten salt possess similar paramagnetic properties to those obtained by other methods, which demonstrates the capability for the facile synthesis of lanthanide borides with controllable stoichiometry and morphology in molten salt under mild conditions.
中文翻译:

具有可控化学计量和形态的硼化熔融盐合成
硼化exhibit具有独特的电和磁性能,在许多领域都有潜在的应用。然而,通过简单的合成方法获得具有可控的化学计量和形态的结晶硼化still仍然具有挑战性。在这里,我们报告了通过卤化mar和NaBH 4在熔融的LiCl-KCl中的反应,一锅法合成两个硼化phase相。通过改变前体比和反应时间来控制化学计量和形态。结晶SmB 4具有直径约为60-90 nm的棒状结构,并且SmB 6获得了约50-100 nm的纳米立方体。根据XRD和SEM结果,SmB 4以Sm / B比为1:3的纳米棒从最初形成的SmB 4纳米膜演化而来,而以Sm / B比为1:10的SmB 6纳米立方的形成是由SmB 4介导的。从膜状SmB 4到立方SmB 6的转变最可能是由于在过量的NaBH 4存在下将Sm 3+还原为Sm 2+而将SmB 4纳米晶体破坏的结果,这与氧化变化是一致的XANES分析得出的状态。SmB 4纳米棒和SmB 6 在熔融盐中合成的纳米立方体具有与通过其他方法获得的相似的顺磁性能,这表明在温和条件下在熔融盐中易于合成化学计量和形态可控的镧系元素硼化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号