当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Double-Phase Heterostructure within Fe-Doped Cu2–xS Quantum Dots with Boosted Electrocatalytic Nitrogen Reduction
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-02-11 , DOI: 10.1021/acssuschemeng.0c08708 Dongxu Zhang 1 , Yanhong Liu 1 , Baodong Mao 1 , Haitao Li 2 , Tianyao Jiang 1 , Dongqi Zhang 1 , Weixuan Dong 1 , Weidong Shi 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-02-11 , DOI: 10.1021/acssuschemeng.0c08708 Dongxu Zhang 1 , Yanhong Liu 1 , Baodong Mao 1 , Haitao Li 2 , Tianyao Jiang 1 , Dongqi Zhang 1 , Weixuan Dong 1 , Weidong Shi 1
Affiliation
|
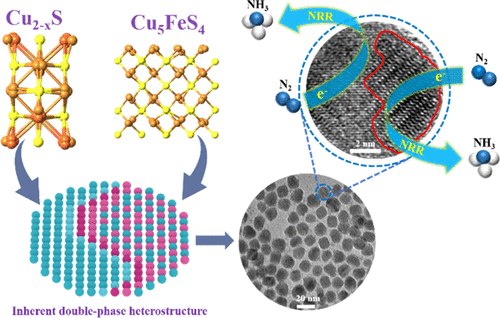
Electrochemical nitrogen reduction reaction (NRR) is considered as one of the most promising methods for NH3 synthesis under room temperature and ambient pressure. A grand challenge of NRR is the development of efficient electrocatalysts, for which the delicate nanostructuring of catalysts plays an important role. Herein, a series of Fe-doped Cu2–xS quantum dots (QDs) are synthesized with multiple active sites and interface engineering, in which the double-phase heterostructure plays a key role for boosting NRR activity. The yield of NH3 was obviously improved with the increase of Fe content from 0 to 3% but started to decrease with Fe from 3 to 9%. The optimized Fe3%–Cu2–xS QDs show an outstanding NH3 yield of 26.4 μg h–1 mg–1cat at −0.7 V (vs the reversible hydrogen electrode), which is 5 times higher than that of Cu2–xS QDs. More importantly, we observed that the highest NRR activity in Fe3%–Cu2–xS QDs was ascribed to the formation of an inherent double-phase heterostructure of Cu2–xS/Cu5FeS4, whereas the complete conversion to single-phase Cu5FeS4 with increased Fe doping (9%) resulted in the activity decrease. Further, N2 temperature-programmed desorption and electrochemical impedance spectra characterizations confirm the stronger chemical adsorption of N2 and faster charge transfer in the Cu2–xS/Cu5FeS4 QDs. A plausible mechanism was proposed for the double-phase Cu2–xS/Cu5FeS4 heterostructure, where the interface provides efficient charge transfer and more active sites of Cu, Fe, and S for the synergetic adsorption and activation of N2. Our work provides a simple strategy for the design of NRR electrocatalysts, which may also bring new inspiration for the preparation of the inherent double-phase heterostructure within other doped QDs.
中文翻译:

增强电催化氮还原作用的Fe掺杂Cu 2 – x S量子点中的双相异质结构
电化学氮还原反应(NRR)被认为是在室温和环境压力下合成NH 3的最有希望的方法之一。NRR的一大挑战是开发高效的电催化剂,为此,催化剂的精细纳米结构起着重要作用。在此,合成了一系列具有多个活性位和界面工程的Fe掺杂Cu 2 – x S量子点(QD),其中双相异质结构在提高NRR活性中起关键作用。随着Fe含量从0%增加到3%,NH 3的收率明显提高,但随着Fe含量从3%增加到9%,NH 3的收率开始下降。优化的Fe 3% –Cu 2– xS QD在-0.7 V(相对于可逆氢电极)下显示出26.4μgh –1 mg –1 cat的出色NH 3产量,是Cu 2- x S QD的5倍。更重要的是,我们观察到Fe 3% –Cu 2– x S QDs中最高的NRR活性归因于Cu 2– x S / Cu 5 FeS 4固有的双相异质结构的形成,而完全转化为Fe掺杂增加(9%)的单相Cu 5 FeS 4导致活性降低。此外,N 2程序升温程序的脱附和电化学阻抗谱表征证实了N 2的更强化学吸附和Cu 2– x S / Cu 5 FeS 4 QD中更快的电荷转移。对于双相Cu 2 – x S / Cu 5 FeS 4异质结构,提出了一个合理的机制,该界面提供了有效的电荷转移以及Cu,Fe和S的更多活性位,以协同吸附和活化N 2。。我们的工作为NRR电催化剂的设计提供了一种简单的策略,这也可能为其他掺杂QD中固有的双相异质结构的制备带来新的启示。
更新日期:2021-02-22
中文翻译:

增强电催化氮还原作用的Fe掺杂Cu 2 – x S量子点中的双相异质结构
电化学氮还原反应(NRR)被认为是在室温和环境压力下合成NH 3的最有希望的方法之一。NRR的一大挑战是开发高效的电催化剂,为此,催化剂的精细纳米结构起着重要作用。在此,合成了一系列具有多个活性位和界面工程的Fe掺杂Cu 2 – x S量子点(QD),其中双相异质结构在提高NRR活性中起关键作用。随着Fe含量从0%增加到3%,NH 3的收率明显提高,但随着Fe含量从3%增加到9%,NH 3的收率开始下降。优化的Fe 3% –Cu 2– xS QD在-0.7 V(相对于可逆氢电极)下显示出26.4μgh –1 mg –1 cat的出色NH 3产量,是Cu 2- x S QD的5倍。更重要的是,我们观察到Fe 3% –Cu 2– x S QDs中最高的NRR活性归因于Cu 2– x S / Cu 5 FeS 4固有的双相异质结构的形成,而完全转化为Fe掺杂增加(9%)的单相Cu 5 FeS 4导致活性降低。此外,N 2程序升温程序的脱附和电化学阻抗谱表征证实了N 2的更强化学吸附和Cu 2– x S / Cu 5 FeS 4 QD中更快的电荷转移。对于双相Cu 2 – x S / Cu 5 FeS 4异质结构,提出了一个合理的机制,该界面提供了有效的电荷转移以及Cu,Fe和S的更多活性位,以协同吸附和活化N 2。。我们的工作为NRR电催化剂的设计提供了一种简单的策略,这也可能为其他掺杂QD中固有的双相异质结构的制备带来新的启示。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号