当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanisms during Atomic Layer Deposition of AlF3 Using Al(CH3)3 and SF6 Plasma
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-02-10 , DOI: 10.1021/acs.jpcc.0c10695
Martijn F. J. Vos 1 , Harm C. M. Knoops 1, 2 , Wilhelmus M. M. Kessels 1 , Adriaan J. M. Mackus 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-02-10 , DOI: 10.1021/acs.jpcc.0c10695
Martijn F. J. Vos 1 , Harm C. M. Knoops 1, 2 , Wilhelmus M. M. Kessels 1 , Adriaan J. M. Mackus 1
Affiliation
|
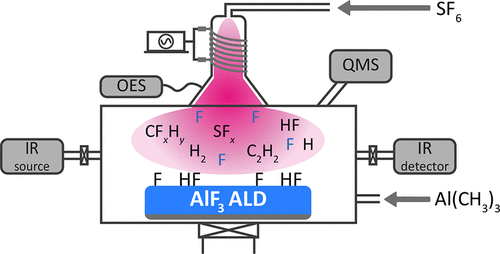
Metal fluorides generally demonstrate a wide band gap and a low refractive index, and they are commonly employed in optics and optoelectronics. Recently, an SF6 plasma was introduced as a novel co-reactant for the atomic layer deposition (ALD) of metal fluorides. In this work, the reaction mechanisms underlying the ALD of fluorides using a fluorine-containing plasma are investigated, considering aluminum fluoride (AlF3) ALD from Al(CH3)3 and an SF6 plasma as a model system. Surface infrared spectroscopy studies indicated that Al(CH3)3 reacts with the surface in a ligand-exchange reaction by accepting F from the AlF3 film and forming CH3 surface groups. It was found that at low deposition temperatures Al(CH3)3 also reacts with HF surface species. These HF species are formed during the SF6 plasma exposure and were detected both at the surface and in the gas phase using infrared spectroscopy and quadrupole mass spectrometry (QMS), respectively. Furthermore, QMS and optical emission spectroscopy (OES) measurements showed that CH4 and CHyF4–y (y ≤ 3) species are the main reaction products during the SF6 plasma exposure. The CH4 release is explained by the reaction of CH3 ligands with HF, while CHyF4–y species originate from the interaction of the SF6 plasma with CH3 ligands. At high temperatures, a transition from AlF3 deposition to Al2O3 etching was observed using infrared spectroscopy. The obtained insights indicate a reaction pathway where F radicals from the SF6 plasma eliminate the CH3 ligands remaining after precursor dosing and where F radicals are simultaneously responsible for the fluorination reaction. The understanding of the reaction mechanisms during AlF3 growth can help in developing ALD processes for other metal fluorides using a fluorine-containing plasma as the co-reactant as well as atomic layer etching (ALE) processes involving surface fluorination.
中文翻译:

Al(CH 3)3和SF 6等离子体沉积AlF 3的反应机理
金属氟化物通常显示出宽带隙和低折射率,并且它们通常用于光学和光电子学中。最近,SF 6等离子体作为一种新型的共反应物被引入,用于金属氟化物的原子层沉积(ALD)。在这项工作中,考虑了以Al(CH 3)3和SF 6等离子体为基础的氟化铝(AlF 3)ALD,研究了使用含氟等离子体的氟化物ALD的反应机理。表面红外光谱研究表明,Al(CH 3)3通过接受AlF 3中的F与配体交换反应与表面反应。膜并形成CH 3表面基团。发现在低沉积温度下,Al(CH 3)3也与HF表面物质反应。这些HF物质是在SF 6等离子体暴露期间形成的,分别使用红外光谱和四极质谱(QMS)在表面和气相中检测到。此外,QMS和光学发射光谱(OES)测量结果表明,CH 4和CH ÿ ˚F 4- ý(Ý ≤3)种类是SF中的主要反应产物6等离子体暴露。CH 4的释放可以通过CH 3的反应来解释CH y F 4– y物种起源于SF 6血浆与CH 3配体的相互作用。在高温下,使用红外光谱观察到从AlF 3沉积到Al 2 O 3蚀刻的转变。所获得的见解表明反应路径,其中SF 6等离子体中的F自由基消除了前体加料后残留的CH 3配体,并且其中F自由基同时负责氟化反应。对AlF 3期间反应机理的理解 生长可以帮助开发其他含氟等离子体作为共反应剂的其他金属氟化物的ALD工艺以及涉及表面氟化的原子层蚀刻(ALE)工艺。
更新日期:2021-02-25
中文翻译:

Al(CH 3)3和SF 6等离子体沉积AlF 3的反应机理
金属氟化物通常显示出宽带隙和低折射率,并且它们通常用于光学和光电子学中。最近,SF 6等离子体作为一种新型的共反应物被引入,用于金属氟化物的原子层沉积(ALD)。在这项工作中,考虑了以Al(CH 3)3和SF 6等离子体为基础的氟化铝(AlF 3)ALD,研究了使用含氟等离子体的氟化物ALD的反应机理。表面红外光谱研究表明,Al(CH 3)3通过接受AlF 3中的F与配体交换反应与表面反应。膜并形成CH 3表面基团。发现在低沉积温度下,Al(CH 3)3也与HF表面物质反应。这些HF物质是在SF 6等离子体暴露期间形成的,分别使用红外光谱和四极质谱(QMS)在表面和气相中检测到。此外,QMS和光学发射光谱(OES)测量结果表明,CH 4和CH ÿ ˚F 4- ý(Ý ≤3)种类是SF中的主要反应产物6等离子体暴露。CH 4的释放可以通过CH 3的反应来解释CH y F 4– y物种起源于SF 6血浆与CH 3配体的相互作用。在高温下,使用红外光谱观察到从AlF 3沉积到Al 2 O 3蚀刻的转变。所获得的见解表明反应路径,其中SF 6等离子体中的F自由基消除了前体加料后残留的CH 3配体,并且其中F自由基同时负责氟化反应。对AlF 3期间反应机理的理解 生长可以帮助开发其他含氟等离子体作为共反应剂的其他金属氟化物的ALD工艺以及涉及表面氟化的原子层蚀刻(ALE)工艺。

































 京公网安备 11010802027423号
京公网安备 11010802027423号