Molecular Therapy: Oncology ( IF 5.3 ) Pub Date : 2021-02-10 , DOI: 10.1016/j.omto.2021.02.006 Erkko Ylösmäki , Leena Ylösmäki , Manlio Fusciello , Beatriz Martins , Petra Ahokas , Hanne Cojoc , Arttu Uoti , Sara Feola , Anna Kreutzman , Tuuli Ranki , Julia Karbach , Tapani Viitala , Petri Priha , Elke Jäger , Sari Pesonen , Vincenzo Cerullo
|
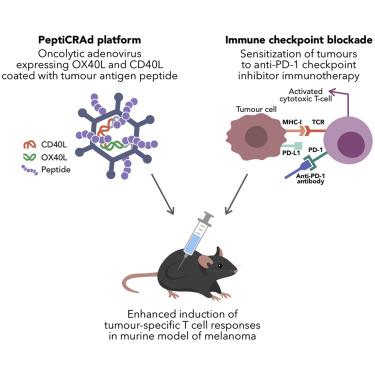
Oncolytic viruses (OVs) have been shown to induce anti-cancer immunity and enhance cancer immunotherapies, such as immune checkpoint inhibitor therapies. OV therapies can be further improved by arming OVs with immunostimulatory molecules, including various cytokines or chemokines. Here, we have developed a novel adenovirus encoding two immunostimulatory molecules: cluster of differentiation 40 ligand (CD40L) and tumor necrosis factor receptor superfamily member 4 ligand (OX40L). This novel virus, designated VALO-D102, is designed to activate both innate and adaptive immune responses against tumors. CD40L affects the innate side by licensing antigen-presenting cells to drive CD8+ T cell responses, and OX40L increases clonal expansion and survival of CD8+ T cells and formation of a larger pool of memory T cells. VALO-D102 and its murine surrogate VALO-mD901, expressing murine OX40L and CD40L, were used in our previously developed PeptiCRAd cancer vaccine platform. Intratumoral administration of PeptiCRAd significantly increased tumor-specific T cell responses, reduced tumor growth, and induced systemic anti-cancer immunity in two mouse models of melanoma. In addition, PeptiCRAd therapy, in combination with anti-PD-1 immune checkpoint inhibitor therapy, significantly improved tumor growth control as compared to either monotherapy alone.
中文翻译:

PeptiCRAd癌症疫苗平台中使用的新型OX40配体和表达CD40配体的溶瘤腺病毒的表征
溶瘤病毒(OVs)已显示出可诱导抗癌免疫力并增强癌症免疫疗法,例如免疫检查点抑制剂疗法。通过用免疫刺激分子(包括各种细胞因子或趋化因子)武装OV,可以进一步改善OV疗法。在这里,我们已经开发出一种新型的腺病毒,它编码两个免疫刺激分子:分化簇40配体(CD40L)和肿瘤坏死因子受体超家族成员4配体(OX40L)。这种新型病毒称为VALO-D102,旨在激活针对肿瘤的先天性和适应性免疫应答。CD40L通过许可抗原呈递细胞驱动CD8 + T细胞反应来影响先天性,而OX40L增加CD8 +的克隆扩增和存活率。T细胞并形成更大的记忆T细胞池。表达鼠OX40L和CD40L的VALO-D102及其鼠替代物VALO-mD901被用于我们先前开发的PeptiCRAd癌症疫苗平台中。在两个黑色素瘤小鼠模型中,对PeptiCRAd进行瘤内给药可显着提高肿瘤特异性T细胞应答,降低肿瘤生长并诱导全身性抗癌免疫。此外,与任何一种单独疗法相比,PeptiCRAd疗法与抗PD-1免疫检查点抑制剂疗法的结合均显着改善了肿瘤的生长控制。































 京公网安备 11010802027423号
京公网安备 11010802027423号