当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complexation of triangular silver(I) or copper(I) nitropyrazolates with dibenzothiophenes having potential use in adsorptive desulfurization
Dalton Transactions ( IF 3.5 ) Pub Date : 2021-2-2 , DOI: 10.1039/d0dt04037a
Lin Yang 1, 2, 3, 4, 5 , Lihong Wang 5, 6, 7, 8 , Xingpu Lv 1, 2, 3, 4, 5 , Jing-Huo Chen 1, 2, 3, 4, 5 , Yang Wang 5, 6, 7, 8 , Guang Yang 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2021-2-2 , DOI: 10.1039/d0dt04037a
Lin Yang 1, 2, 3, 4, 5 , Lihong Wang 5, 6, 7, 8 , Xingpu Lv 1, 2, 3, 4, 5 , Jing-Huo Chen 1, 2, 3, 4, 5 , Yang Wang 5, 6, 7, 8 , Guang Yang 1, 2, 3, 4, 5
Affiliation
|
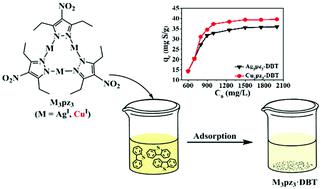
Triangular silver(I) and copper(I) 3,5-diethyl-4-nitropyrazolates (abbreviated as [Ag(denpz)]3 or Ag3pz3, and [Cu(denpz)]3 or Cu3pz3), as well as their adducts with dibenzothiophene (DBT), 4,6-dimethyldibenzothiophene (DMDBT) and benzothiophene (BT), have been prepared and characterized by a series of techniques. X-ray analyses show that these adducts are stabilized by M⋯S, M⋯C contacts and π⋯π stacking interactions. NMR measurements and theoretical calculations indicate that the intensity of interaction between the metal complexes and dibenzothiophenes follows the trend: Ag3pz3–DMDBT > Ag3pz3–DBT > Cu3pz3–DMDBT > Cu3pz3–DBT, which can be understood on the basis of a weak interaction between π-acid (Ag3pz3 or Cu3pz3) and π-base (DBT/DMDBT). Both complexes show good adsorptive ability and reusability toward the removal of DBT and DMDBT from model oil (n-octane), with the maximum adsorption capacity at room temperature being 39 mg S (DMDBT) per g Cu3pz3, 34 mg S (DMDBT) per g Ag3pz3, 40 mg S (DBT) per g Cu3pz3, 36 mg S (DBT) per g Ag3pz3, respectively. Compared to Ag3pz3, Cu3pz3 exhibits higher adsorptive capacities for DBT/DMDBT, which has been attributed to its lower molecular mass.
中文翻译:

三角形吡唑啉银(I)或硝基吡唑铜(I)与二苯并噻吩的络合在吸附脱硫中有潜在用途
3,5-二乙基-4-硝基吡唑酸盐(简称为[Ag(denpz)] 3或Ag 3 pz 3和[Cu(denpz)] 3或Cu 3 pz 3)的三角形银(I)和铜(I),以及它们与二苯并噻吩(DBT),4,6-二甲基二苯并噻吩(DMDBT)和苯并噻吩(BT)的加合物已经制备并通过一系列技术进行了表征。X射线分析表明,这些加合物被M⋯S,M⋯C接触和π⋯π堆积相互作用所稳定。NMR测量和理论计算表明,金属络合物与二苯并噻吩之间的相互作用强度遵循以下趋势:Ag 3pz 3 –DMDBT> Ag 3 pz 3 –DBT> Cu 3 pz 3 –DMDBT> Cu 3 pz 3 –DBT,可以基于π-酸(Ag 3 pz 3或Cu 3 pz)之间的弱相互作用来理解3)和π基(DBT / DMDBT)。两种配合物对从模型油(正辛烷)中去除DBT和DMDBT均显示出良好的吸附能力和可重复使用性,室温下的最大吸附容量为39 g S(DMDBT)/ g Cu 3 pz 3每克Ag 3 pz 3分别为34 mg S(DMDBT),每克Cu 3 pz 3 40 mg S(DBT)和每克Ag 3 pz 3 36 mg S(DBT)。与Ag 3 pz 3相比,Cu 3 pz 3对DBT / DMDBT的吸附能力更高,这归因于其较低的分子量。
更新日期:2021-02-08
中文翻译:

三角形吡唑啉银(I)或硝基吡唑铜(I)与二苯并噻吩的络合在吸附脱硫中有潜在用途
3,5-二乙基-4-硝基吡唑酸盐(简称为[Ag(denpz)] 3或Ag 3 pz 3和[Cu(denpz)] 3或Cu 3 pz 3)的三角形银(I)和铜(I),以及它们与二苯并噻吩(DBT),4,6-二甲基二苯并噻吩(DMDBT)和苯并噻吩(BT)的加合物已经制备并通过一系列技术进行了表征。X射线分析表明,这些加合物被M⋯S,M⋯C接触和π⋯π堆积相互作用所稳定。NMR测量和理论计算表明,金属络合物与二苯并噻吩之间的相互作用强度遵循以下趋势:Ag 3pz 3 –DMDBT> Ag 3 pz 3 –DBT> Cu 3 pz 3 –DMDBT> Cu 3 pz 3 –DBT,可以基于π-酸(Ag 3 pz 3或Cu 3 pz)之间的弱相互作用来理解3)和π基(DBT / DMDBT)。两种配合物对从模型油(正辛烷)中去除DBT和DMDBT均显示出良好的吸附能力和可重复使用性,室温下的最大吸附容量为39 g S(DMDBT)/ g Cu 3 pz 3每克Ag 3 pz 3分别为34 mg S(DMDBT),每克Cu 3 pz 3 40 mg S(DBT)和每克Ag 3 pz 3 36 mg S(DBT)。与Ag 3 pz 3相比,Cu 3 pz 3对DBT / DMDBT的吸附能力更高,这归因于其较低的分子量。































 京公网安备 11010802027423号
京公网安备 11010802027423号