当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Robust alkyl‐bridged bis(N‐heterocyclic carbene)palladium(II) complexes anchored on Merrifield's resin as active catalysts for the selective synthesis of flavones and alkynones
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-02-07 , DOI: 10.1002/aoc.6195 Waseem Mansour 1 , Mohammed Fettouhi 1 , Qasim Saleem 2 , Bassam El Ali 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-02-07 , DOI: 10.1002/aoc.6195 Waseem Mansour 1 , Mohammed Fettouhi 1 , Qasim Saleem 2 , Bassam El Ali 1
Affiliation
|
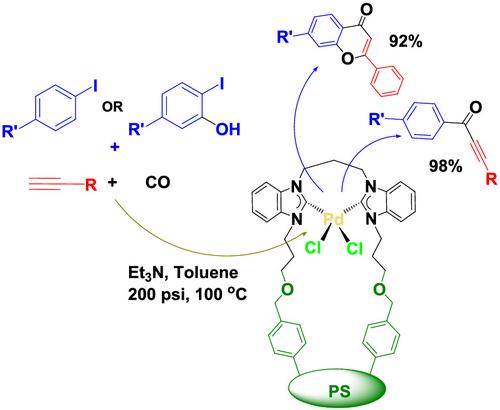
Highly active and efficient propylene‐bridged bis(N‐heterocyclic carbene)palladium(II) complexes covalently anchored on Merrifield's resin were synthesized and characterized using various physical and spectroscopic techniques. The two anchored Pd(II) complexes consist of the system: Merrifield's resin‐linker‐bis(NHC)Pd(II), the linkers being benzyl and benzyl‐O‐(CH2)3 for (Pd‐NHC1@M) and (Pd‐NHC2@M), respectively. The short linker anchored bis‐benzimidazolium ligand precursor (PBBI‐1@M) was synthesized via direct carbon–nitrogen alkylation of a propylene‐bridged bis(benzimidazole) (PBBI‐1) by Merrifield's resin chlorobenzyl group. The longer linker anchored bis‐benzimidazolium ligand precursor (PBBI‐2@M) was obtained in a two‐step reaction involving first alkylation of (PBBI‐1) with 3‐chloro‐1‐propanol followed by a nucleophilic substitution at Merrifield's resin chlorobenzyl group. Both supported ligand precursors (PBBI‐1@M and PBBI‐2@M) reacted with palladium acetate to produce the two heterogeneous catalysts (Pd‐NHC1@M) and (Pd‐NHC2@M). 13C NMR palladation shift of the benzimidazole N–C–N (C2) carbon was found very similar in both the liquid NMR spectra of the homogeneous complexes and the CP/MASS spectra of the corresponding covalently anchored complexes. The catalytic activity, stability, and the recycling ability of the supported catalysts have been investigated in the carbonylative Sonogashira coupling reactions of aryl iodides with aryl alkynes and alkyl alkynes and also in the cyclocarbonylative Sonogashira coupling reactions of aryl iodides with aryl alkynes via one pot reactions. The longer linker catalyst Pd‐NHC2@M demonstrated excellent catalytic activity, stability, and very high recycling ability in the two carbonylative coupling reactions. These systems exhibit the hypothesized thermodynamic stability offered by the chelate effect in addition to the strong sigma donor ability of a bis(NHC) ligand system generating electron‐rich palladium centers that favor the oxidative addition step of the aryl halide.
中文翻译:

牢固的烷基桥双(N-杂环卡宾)钯(II)配合物锚固在Merrifield的树脂上,作为活性催化剂选择性合成黄酮和炔酮
使用各种物理和光谱技术合成并表征了共价锚固在Merrifield树脂上的高活性和高效的丙烯桥双(N-杂环卡宾)钯(II)配合物。两个固定的Pd(II)配合物由以下系统组成:Merrifield的树脂-连接基-双(NHC)Pd(II),连接基为(Pd-NHC1 @ M)的苄基和苄基-O-(CH 2)3和(Pd-NHC2 @ M)。短接头锚定的双苯并咪唑配体前体(PBBI-1 @ M)是通过丙烯桥联的双(苯并咪唑)(PBBI-1)的直接碳-氮烷基化反应合成的)由Merrifield's树脂的氯苄基组成。较长的接头锚定双苯并咪唑鎓配体前体(PBBI-2 @ M)是通过两步反应获得的,该过程涉及先将(PBBI-1)与3-氯-1-丙醇进行烷基化,然后在Merrifield的树脂氯苄基上进行亲核取代团体。两种负载的配体前体(PBBI-1 @ M和PBBI-2 @ M)与乙酸钯反应生成两种非均相催化剂(Pd-NHC1 @ M)和(Pd-NHC2 @ M)。13在均相配合物的液相NMR光谱和相应的共价锚定配合物的CP / MASS光谱中,发现苯并咪唑N–C–N(C2)碳的C NMR palpalation移动非常相似。已通过一锅法在芳基碘化物与芳基炔烃和烷基炔烃的羰基化Sonogashira偶联反应以及芳基碘化物与芳基炔烃的环羰基Sonogashira偶联反应中研究了负载型催化剂的催化活性,稳定性和再循环能力。 。较长的连接子催化剂Pd-NHC2 @ M在两个羰基化偶联反应中显示出优异的催化活性,稳定性和很高的再循环能力。这些系统除了具有双(NHC)配体系统强大的sigma供体能力外,还具有螯合效应所提供的假设的热力学稳定性,双(NHC)配体系统会生成富电子的钯中心,从而有利于芳基卤化物的氧化加成步骤。
更新日期:2021-04-14
中文翻译:

牢固的烷基桥双(N-杂环卡宾)钯(II)配合物锚固在Merrifield的树脂上,作为活性催化剂选择性合成黄酮和炔酮
使用各种物理和光谱技术合成并表征了共价锚固在Merrifield树脂上的高活性和高效的丙烯桥双(N-杂环卡宾)钯(II)配合物。两个固定的Pd(II)配合物由以下系统组成:Merrifield的树脂-连接基-双(NHC)Pd(II),连接基为(Pd-NHC1 @ M)的苄基和苄基-O-(CH 2)3和(Pd-NHC2 @ M)。短接头锚定的双苯并咪唑配体前体(PBBI-1 @ M)是通过丙烯桥联的双(苯并咪唑)(PBBI-1)的直接碳-氮烷基化反应合成的)由Merrifield's树脂的氯苄基组成。较长的接头锚定双苯并咪唑鎓配体前体(PBBI-2 @ M)是通过两步反应获得的,该过程涉及先将(PBBI-1)与3-氯-1-丙醇进行烷基化,然后在Merrifield的树脂氯苄基上进行亲核取代团体。两种负载的配体前体(PBBI-1 @ M和PBBI-2 @ M)与乙酸钯反应生成两种非均相催化剂(Pd-NHC1 @ M)和(Pd-NHC2 @ M)。13在均相配合物的液相NMR光谱和相应的共价锚定配合物的CP / MASS光谱中,发现苯并咪唑N–C–N(C2)碳的C NMR palpalation移动非常相似。已通过一锅法在芳基碘化物与芳基炔烃和烷基炔烃的羰基化Sonogashira偶联反应以及芳基碘化物与芳基炔烃的环羰基Sonogashira偶联反应中研究了负载型催化剂的催化活性,稳定性和再循环能力。 。较长的连接子催化剂Pd-NHC2 @ M在两个羰基化偶联反应中显示出优异的催化活性,稳定性和很高的再循环能力。这些系统除了具有双(NHC)配体系统强大的sigma供体能力外,还具有螯合效应所提供的假设的热力学稳定性,双(NHC)配体系统会生成富电子的钯中心,从而有利于芳基卤化物的氧化加成步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号