当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Co‐Catalyzed Asymmetric Intramolecular [3+2] Cycloaddition of Yne‐Alkylidenecyclopropanes and its Reaction Mechanism
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-02-07 , DOI: 10.1002/chem.202100426
Zhixiang Yu 1 , Xiong Xiao 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-02-07 , DOI: 10.1002/chem.202100426
Zhixiang Yu 1 , Xiong Xiao 2
Affiliation
|
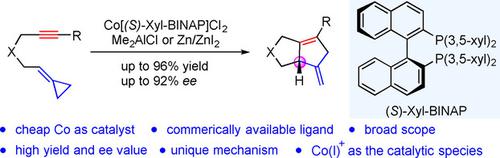
Developing new transition metal‐catalyzed asymmetric cycloadditions for the synthesis of five‐membered carbocycles (FMCs) is a research frontier in reaction development due to the ubiquitous presence of chiral FMCs in various functional molecules. Reported here is our discovery of a highly enantioselective intramolecular [3+2] cycloaddition of yne‐alkylidenecyclopropanes (yne‐ACPs) to bicyclo[3.3.0]octadiene and bicyclo[4.3.0]nonadiene molecules using a cheap Co catalyst and commercially available chiral ligand (S)‐Xyl‐BINAP. This reaction avoids the use of precious Pd and Rh catalysts, which are usually the choices for [3+2] reactions with ACPs. The enantiomeric excess in the present reaction can be up to 92 %. Cationic cobalt(I) species was suggested by experiments as the catalytic species. DFT calculations showed that this [3+2] reaction starts with oxidative cyclometallation of alkyne and ACP, followed by ring opening of the cyclopropyl (CP) group and reductive elimination to form the cycloadduct. This mechanism is different from previous [3+2] reactions of ACPs, which usually start from CP cleavage, not from oxidative cyclization.
中文翻译:

Yne-亚烷基环丙烷的共催化不对称分子内[3 + 2]环加成反应及其机理
由于各种功能分子中普遍存在手性FMC,因此开发用于合成五元碳环(FMC)的新型过渡金属催化的不对称环加成化合物是反应发展的研究前沿。我们在这里报道的发现是使用便宜的Co催化剂,将炔-亚烷基环丙烷(yne-ACP)高度对映选择性的[3 + 2]环内加成成双环[3.3.0]辛二烯和双环[4.3.0]壬二烯分子,并且可以通过商业途径获得手性配体(小号)-Xyl-BINAP。该反应避免了使用贵重的Pd和Rh催化剂,这通常是与ACP进行[3 + 2]反应的选择。本反应中的对映体过量可高达92%。实验表明阳离子钴(I)是催化物种。DFT计算表明,此[3 + 2]反应始于炔烃和ACP的氧化环金属化,然后是环丙基(CP)基团的开环和还原消除,从而形成环加合物。该机制不同于以前的ACP的[3 + 2]反应,后者通常是从CP裂解而不是氧化环化开始的。
更新日期:2021-02-07
中文翻译:

Yne-亚烷基环丙烷的共催化不对称分子内[3 + 2]环加成反应及其机理
由于各种功能分子中普遍存在手性FMC,因此开发用于合成五元碳环(FMC)的新型过渡金属催化的不对称环加成化合物是反应发展的研究前沿。我们在这里报道的发现是使用便宜的Co催化剂,将炔-亚烷基环丙烷(yne-ACP)高度对映选择性的[3 + 2]环内加成成双环[3.3.0]辛二烯和双环[4.3.0]壬二烯分子,并且可以通过商业途径获得手性配体(小号)-Xyl-BINAP。该反应避免了使用贵重的Pd和Rh催化剂,这通常是与ACP进行[3 + 2]反应的选择。本反应中的对映体过量可高达92%。实验表明阳离子钴(I)是催化物种。DFT计算表明,此[3 + 2]反应始于炔烃和ACP的氧化环金属化,然后是环丙基(CP)基团的开环和还原消除,从而形成环加合物。该机制不同于以前的ACP的[3 + 2]反应,后者通常是从CP裂解而不是氧化环化开始的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号