Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-02-08 , DOI: 10.1016/j.bioorg.2021.104715 Cheng-I Lee , Chu-Bin Liao , Chih-Shang Chen , Fen-Ying Cheng , Yu-Hsuan Chung , Yu-Chuan Wang , Sian-Yi Ciou , Wen-Yun Hsueh , Tzu-Hao Lo , Guan-Ru Huang , Hsin-Yi Huang , Chia-Shen Tsai , Yu-Jung Lu , Shih-Hsien Chuang , Jiann-Jyh Huang
|
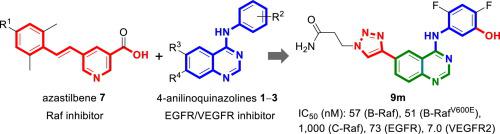
This paper presents the design and synthesis of 4-(3-hydroxyanilino)-6-(1H-1,2,3-triazol-4-yl)quinazolines of scaffold 9 as selective B-Raf/B-RafV600E and potent EGFR/VEGFR2 kinase inhibitors. Total 14 compounds of scaffold 9 having different side chains at the triazolyl group with/without fluoro substituents at the anilino group were synthesized and investigated. Among them, 9m with a 2-carbamoylethyl side chain and C-4′/C-6′ difluoro substituents was the most potent, which selectively inhibited B-Raf (IC50: 57 nM) and B-RafV600E (IC50: 51 nM) over C-Raf (IC50: 1.0 μM). Compound 9m also actively inhibited EGFR (IC50: 73 nM) and VEGFR2 (IC50: 7.0 nM) but not EGFRT790M and PDGFR-β (IC50: >10 μM). Despite having good potency for B-Raf and B-RafV600E in the enzymatic assays, 9m was less active to inhibit melanoma A375 cells which proliferate due to constitutively activated B-Raf600E. The inferior activity of 9m for A375 was similar to that of sorafenib (6), suggesting that 9m might bind to the inactive conformations of B-Raf and B-RafV600E. Docking simulations could thus be performed to reveal the binding poses of 9m in B-Raf, B-RafV600E, and VEGFR2 kinases.
中文翻译:

作为 Raf 激酶抑制剂的 4-苯胺基喹唑啉的设计和合成。第 1 部分。选择性 B-Raf/B-RafV600E 和强效 EGFR/VEGFR2 抑制 4-(3-羟基苯胺)-6-(1H-1,2,3-triazol-4-yl)quinazolines
本文介绍了支架9的 4-(3-羟基苯胺基)-6-(1 H -1,2,3-三唑-4-基)喹唑啉作为选择性 B-Raf/B-Raf V600E和有效的设计和合成EGFR/VEGFR2激酶抑制剂。合成并研究了在三唑基具有/不具有氟取代基的三唑基基团处具有不同侧链的支架9的总共14种化合物并进行了研究。其中,具有 2-氨基甲酰基乙基侧链和 C-4'/C-6' 二氟取代基的9m是最有效的,它选择性地抑制 B-Raf (IC 50 : 57 nM) 和 B-Raf V600E (IC 50 : 51 nM) 超过 C-Raf (IC 50 : 1.0 μM)。化合物9m还积极抑制 EGFR (IC 50 : 73 nM) 和 VEGFR2 (IC 50 : 7.0 nM) 但不抑制EGFR T790M和 PDGFR-β (IC 50 : >10 μM)。尽管在酶促测定中对 B-Raf 和 B-Raf V600E具有良好的效力,但9m对抑制由于组成型激活的 B-Raf 600E而增殖的黑色素瘤 A375 细胞的活性较低。9m对 A375的较差活性类似于索拉非尼 ( 6 ),表明9m可能与 B-Raf 和 B-Raf V600E的无活性构象结合。因此可以进行对接模拟以揭示在 B-Raf、B-Raf V600E和 VEGFR2 激酶中为9m。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号