Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypertrophic cardiomyopathy associated E22K mutation in myosin regulatory light chain decreases calcium-activated tension and stiffness and reduces myofilament Ca2+ sensitivity
The FEBS Journal ( IF 5.5 ) Pub Date : 2021-02-06 , DOI: 10.1111/febs.15753 Jiajia Zhang 1 , Li Wang 1 , Katarzyna Kazmierczak 2 , Hang Yun 1 , Danuta Szczesna-Cordary 2 , Masataka Kawai 3
The FEBS Journal ( IF 5.5 ) Pub Date : 2021-02-06 , DOI: 10.1111/febs.15753 Jiajia Zhang 1 , Li Wang 1 , Katarzyna Kazmierczak 2 , Hang Yun 1 , Danuta Szczesna-Cordary 2 , Masataka Kawai 3
Affiliation
|
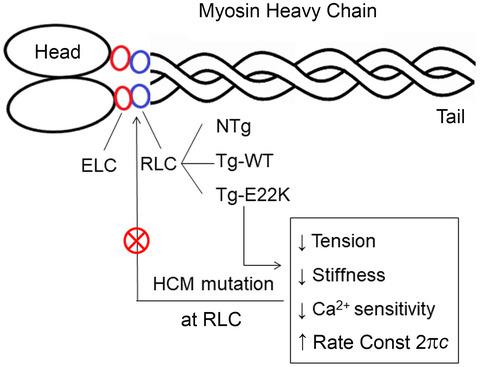
We investigated the mechanisms associated with E22K mutation in myosin regulatory light chain (RLC), found to cause hypertrophic cardiomyopathy (HCM) in humans and mice. Specifically, we characterized the mechanical profiles of papillary muscle fibers from transgenic mice expressing human ventricular RLC wild-type (Tg-WT) or E22K mutation (Tg-E22K). Because the two mouse models expressed different amounts of transgene, the B6SJL mouse line (NTg) was used as an additional control. Mechanical experiments were carried out on Ca2+- and ATP-activated fibers and in rigor. Sinusoidal analysis was performed to elucidate the effect of E22K on tension and stiffness during activation/rigor, tension-pCa, and myosin cross-bridge (CB) kinetics. We found significant reductions in active tension (by 54%) and stiffness (active by 40% and rigor by 54%). A decrease in the Ca2+ sensitivity of tension (by ∆pCa ~ 0.1) was observed in Tg-E22K compared with Tg-WT fibers. The apparent (=measured) rate constant of exponential process B (2πb: force generation step) was not affected by E22K, but the apparent rate constant of exponential process C (2πc: CB detachment step) was faster in Tg-E22K compared with Tg-WT fibers. Both 2πb and 2πc were smaller in NTg than in Tg-WT fibers, suggesting a kinetic difference between the human and mouse RLC. Our results of E22K-induced reduction in myofilament stiffness and tension suggest that the main effect of this mutation was to disturb the interaction of RLC with the myosin heavy chain and impose structural abnormalities in the lever arm of myosin CB. When placed in vivo, the E22K mutation is expected to result in reduced contractility and decreased cardiac output whereby leading to HCM.
中文翻译:

肥厚型心肌病与肌球蛋白调节轻链中的 E22K 突变相关,可降低钙激活的张力和僵硬,并降低肌丝 Ca2+ 敏感性
我们研究了与肌球蛋白调节轻链 (RLC) 中 E22K 突变相关的机制,发现它会导致人类和小鼠肥厚性心肌病 (HCM)。具体而言,我们表征了来自表达人心室 RLC 野生型 (Tg-WT) 或 E22K 突变 (Tg-E22K) 的转基因小鼠的乳头肌纤维的机械特征。由于两种小鼠模型表达的转基因量不同,因此使用 B6SJL 小鼠品系 (NTg) 作为额外的对照。对 Ca 2+进行机械实验- 和 ATP 激活的纤维和严格的。进行正弦分析以阐明 E22K 在激活/严格、张力-pCa 和肌球蛋白横桥 (CB) 动力学过程中对张力和刚度的影响。我们发现主动张力(降低 54%)和刚度(主动降低 40%,刚性降低 54%)显着降低。与 Tg-WT 纤维相比,在 Tg-E22K 中观察到的 Ca 2+张力敏感性降低(ΔpCa ~ 0.1)。指数过程B(2π b:力生成步骤)的表观(=测量)速率常数不受 E22K 的影响,但与Tg-E22K 相比,指数过程C(2π c:CB 分离步骤)的表观速率常数更快用 Tg-WT 纤维。都 2π b和 2π c在 NTg 中比在 Tg-WT 纤维中小,表明人和小鼠 RLC 之间存在动力学差异。我们的 E22K 诱导的肌丝刚度和张力降低的结果表明,这种突变的主要影响是干扰 RLC 与肌球蛋白重链的相互作用,并在肌球蛋白 CB 的杠杆臂中施加结构异常。当置于体内时,预计 E22K 突变会导致收缩力降低和心输出量减少,从而导致 HCM。
更新日期:2021-02-06
中文翻译:

肥厚型心肌病与肌球蛋白调节轻链中的 E22K 突变相关,可降低钙激活的张力和僵硬,并降低肌丝 Ca2+ 敏感性
我们研究了与肌球蛋白调节轻链 (RLC) 中 E22K 突变相关的机制,发现它会导致人类和小鼠肥厚性心肌病 (HCM)。具体而言,我们表征了来自表达人心室 RLC 野生型 (Tg-WT) 或 E22K 突变 (Tg-E22K) 的转基因小鼠的乳头肌纤维的机械特征。由于两种小鼠模型表达的转基因量不同,因此使用 B6SJL 小鼠品系 (NTg) 作为额外的对照。对 Ca 2+进行机械实验- 和 ATP 激活的纤维和严格的。进行正弦分析以阐明 E22K 在激活/严格、张力-pCa 和肌球蛋白横桥 (CB) 动力学过程中对张力和刚度的影响。我们发现主动张力(降低 54%)和刚度(主动降低 40%,刚性降低 54%)显着降低。与 Tg-WT 纤维相比,在 Tg-E22K 中观察到的 Ca 2+张力敏感性降低(ΔpCa ~ 0.1)。指数过程B(2π b:力生成步骤)的表观(=测量)速率常数不受 E22K 的影响,但与Tg-E22K 相比,指数过程C(2π c:CB 分离步骤)的表观速率常数更快用 Tg-WT 纤维。都 2π b和 2π c在 NTg 中比在 Tg-WT 纤维中小,表明人和小鼠 RLC 之间存在动力学差异。我们的 E22K 诱导的肌丝刚度和张力降低的结果表明,这种突变的主要影响是干扰 RLC 与肌球蛋白重链的相互作用,并在肌球蛋白 CB 的杠杆臂中施加结构异常。当置于体内时,预计 E22K 突变会导致收缩力降低和心输出量减少,从而导致 HCM。































 京公网安备 11010802027423号
京公网安备 11010802027423号