Tetrahedron ( IF 2.1 ) Pub Date : 2021-02-06 , DOI: 10.1016/j.tet.2021.131993 Hiroki Naruto , Hideo Togo
|
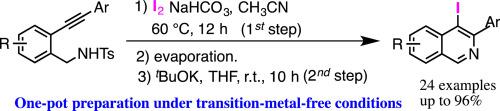
Treatment of N-(o-arylethynyl)benzyl p-toluenesulfonamides with molecular iodine in the presence of NaHCO3 at 60 °C, followed by the reaction with tBuOK at room temperature gave 3-aryl-4-iodoisoquinolines in good yields. 4-Iodo-3-phenylisoquinoline, which is one of the obtained 3-aryl-4-iodoisoquinolines, was further transformed into isoquinoline derivatives smoothly. The present approach is a novel one-pot method for the preparation of 3-aryl-4-iodoisoquinolines from N-(o-arylethynyl)benzyl p-toluenesulfonamides under transition-metal-free conditions. © 2021 Elsevier Science. All rights reserved.
中文翻译:

从N-(o-芳基乙炔基)苄基对甲苯磺酰胺与碘和碱轻松制备3-芳基-4-碘异喹啉
在60℃下在NaHCO 3存在下用分子碘处理N-(邻-芳基乙炔基)苄基对甲苯磺酰胺,然后在室温下与t BuOK反应,以良好的收率得到3-芳基-4-碘异喹啉。将得到的3-芳基-4-碘异喹啉之一的4-碘-3-苯基异喹啉进一步平滑地转化为异喹啉衍生物。本方法是在无过渡金属的条件下由N-(邻-芳基乙炔基)苄基对甲苯磺酰胺制备3-芳基-4-碘异喹啉的一锅法。©2021爱思唯尔科学。版权所有。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号