当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of Tubulysin Analogues, Linker-Drugs, and Antibody–Drug Conjugates, Insights into Structure–Activity Relationships, and Tubulysin–Tubulin Binding Derived from X-ray Crystallographic Analysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-02-05 , DOI: 10.1021/acs.joc.0c02755 K C Nicolaou 1 , Saiyong Pan 1 , Kiran K Pulukuri 1 , Qiuji Ye 1 , Stephan Rigol 1 , Rohan D Erande 1 , Dionisios Vourloumis 1, 2 , Bogusław P Nocek 3 , Stefan Munneke 4 , Joseph Lyssikatos 4 , Amanda Valdiosera 4 , Christine Gu 4 , Baiwei Lin 4 , Hetal Sarvaiaya 4 , Jose Trinidad 4 , Joseph Sandoval 4 , Christina Lee 4 , Mikhail Hammond 4 , Monette Aujay 4 , Nicole Taylor 4 , Marybeth Pysz 4 , James W Purcell 4 , Julia Gavrilyuk 4
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-02-05 , DOI: 10.1021/acs.joc.0c02755 K C Nicolaou 1 , Saiyong Pan 1 , Kiran K Pulukuri 1 , Qiuji Ye 1 , Stephan Rigol 1 , Rohan D Erande 1 , Dionisios Vourloumis 1, 2 , Bogusław P Nocek 3 , Stefan Munneke 4 , Joseph Lyssikatos 4 , Amanda Valdiosera 4 , Christine Gu 4 , Baiwei Lin 4 , Hetal Sarvaiaya 4 , Jose Trinidad 4 , Joseph Sandoval 4 , Christina Lee 4 , Mikhail Hammond 4 , Monette Aujay 4 , Nicole Taylor 4 , Marybeth Pysz 4 , James W Purcell 4 , Julia Gavrilyuk 4
Affiliation
|
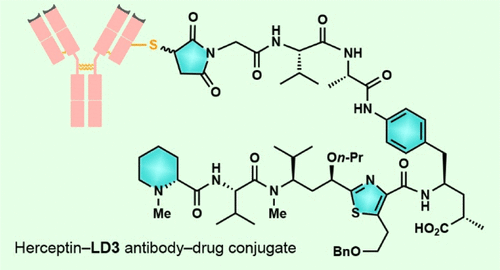
Molecular design, synthesis, and biological evaluation of tubulysin analogues, linker-drugs, and antibody–drug conjugates are described. Among the new discoveries reported is the identification of new potent analogues within the tubulysin family that carry a C11 alkyl ether substituent, rather than the usual ester structural motif at that position, a fact that endows the former with higher plasma stability than that of the latter. Also described herein are X-ray crystallographic analysis studies of two tubulin–tubulysin complexes formed within the α/β interface between two tubulin heterodimers and two highly potent tubulysin analogues, one of which exhibited a different binding mode to the one previously reported for tubulysin M. The X-ray crystallographic analysis-derived new insights into the binding modes of these tubulysin analogues explain their potencies and provide inspiration for further design, synthesis, and biological investigations within this class of antitumor agents. A number of these analogues were conjugated as payloads with appropriate linkers at different sites allowing their attachment onto targeting antibodies for cancer therapies. A number of such antibody–drug conjugates were constructed and tested, both in vivo and in vitro, leading to the identification of at least one promising ADC (Herceptin–LD3), warranting further investigations.
中文翻译:

从X射线晶体学分析中制备和研究Tubulysin类似物,接头药物和抗体-药物缀合物的结构,活性和结构-活性关系以及Tubulysin-管蛋白结合的设计,合成和生物学评估。
描述了微管蛋白溶血素类似物,接头药物和抗体药物偶联物的分子设计,合成和生物学评估。在报道的新发现中,鉴定出微管溶素家族中带有C11烷基醚取代基的新有效类似物,而不是该位置的常规酯结构基序,这一事实使前者具有比后者更高的血浆稳定性。 。本文还描述了在两种微管蛋白异二聚体和两种高效微管蛋白类似物之间的α/β界面内形成的两种微管蛋白-微管蛋白溶解复合物的X射线晶体分析研究,其中一种与先前报道的微管蛋白M的结合模式不同。 。X射线晶体学分析得出了这些微管蛋白类似物的结合模式的新见解,解释了它们的潜能,并为此类抗肿瘤药的进一步设计,合成和生物学研究提供了启发。许多这样的类似物在不同部位与有效连接物缀合为有效负载,从而使其附着在靶向抗体上进行癌症治疗。在体内和体外构建并测试了许多此类抗体-药物偶联物,从而鉴定出至少一种有希望的ADC(Herceptin- 许多这样的类似物在不同部位与有效连接物缀合为有效负载,从而使其附着在靶向抗体上进行癌症治疗。在体内和体外构建并测试了许多此类抗体-药物偶联物,从而鉴定出至少一种有希望的ADC(Herceptin- 许多这样的类似物在不同部位与有效连接物缀合为有效负载,从而使其附着在靶向抗体上进行癌症治疗。在体内和体外构建并测试了许多此类抗体-药物偶联物,从而鉴定出至少一种有希望的ADC(Herceptin-LD3),有待进一步调查。
更新日期:2021-02-19
中文翻译:

从X射线晶体学分析中制备和研究Tubulysin类似物,接头药物和抗体-药物缀合物的结构,活性和结构-活性关系以及Tubulysin-管蛋白结合的设计,合成和生物学评估。
描述了微管蛋白溶血素类似物,接头药物和抗体药物偶联物的分子设计,合成和生物学评估。在报道的新发现中,鉴定出微管溶素家族中带有C11烷基醚取代基的新有效类似物,而不是该位置的常规酯结构基序,这一事实使前者具有比后者更高的血浆稳定性。 。本文还描述了在两种微管蛋白异二聚体和两种高效微管蛋白类似物之间的α/β界面内形成的两种微管蛋白-微管蛋白溶解复合物的X射线晶体分析研究,其中一种与先前报道的微管蛋白M的结合模式不同。 。X射线晶体学分析得出了这些微管蛋白类似物的结合模式的新见解,解释了它们的潜能,并为此类抗肿瘤药的进一步设计,合成和生物学研究提供了启发。许多这样的类似物在不同部位与有效连接物缀合为有效负载,从而使其附着在靶向抗体上进行癌症治疗。在体内和体外构建并测试了许多此类抗体-药物偶联物,从而鉴定出至少一种有希望的ADC(Herceptin- 许多这样的类似物在不同部位与有效连接物缀合为有效负载,从而使其附着在靶向抗体上进行癌症治疗。在体内和体外构建并测试了许多此类抗体-药物偶联物,从而鉴定出至少一种有希望的ADC(Herceptin- 许多这样的类似物在不同部位与有效连接物缀合为有效负载,从而使其附着在靶向抗体上进行癌症治疗。在体内和体外构建并测试了许多此类抗体-药物偶联物,从而鉴定出至少一种有希望的ADC(Herceptin-LD3),有待进一步调查。































 京公网安备 11010802027423号
京公网安备 11010802027423号