当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biocomposite hydrogel beads from glutaraldehyde-crosslinked phytochemicals in alginate for effective removal of methylene blue
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-02-05 , DOI: 10.1016/j.molliq.2021.115579 Andreas Andreas , Zefanya Gerald Winata , Shella Permatasari Santoso , Artik Elisa Angkawijaya , Maria Yuliana , Felycia Edi Soetaredjo , Suryadi Ismadji , Hsien-Yi Hsu , Alchris Woo Go , Yi-Hsu Ju
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-02-05 , DOI: 10.1016/j.molliq.2021.115579 Andreas Andreas , Zefanya Gerald Winata , Shella Permatasari Santoso , Artik Elisa Angkawijaya , Maria Yuliana , Felycia Edi Soetaredjo , Suryadi Ismadji , Hsien-Yi Hsu , Alchris Woo Go , Yi-Hsu Ju
|
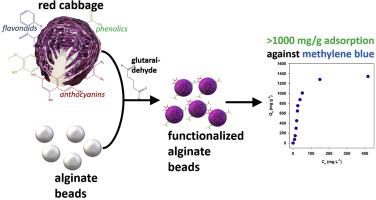
Phytochemicals, i.e., flavonoids, phenolics, and anthocyanin, extracted from red cabbage, were crosslinked with alginate to prepare biocomposite hydrogel beads (BHB). The preparation of BHB involved three consecutive steps: (1) extraction and solvent reduction of phytochemicals from red cabbage, (2) crosslinking of phytochemicals into alginate matrix using glutaraldehyde, and (3) formation of the hydrogel beads in CaCl2 solution. The resulting BHB sorbents were characterized using scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) analyses. The cross-section structure of the BHB was confirmed from the SEM images. The alteration of FTIR peaks implied the success of the crosslinking of phytochemical compounds into the alginate. The adsorption equilibrium and kinetic studies of BHB were conducted using basic blue 9 (BB9) as the model adsorbate. FTIR characterization of the BHB post-adsorption reveals the functional groups of the adsorbent involved in the dye adsorption. The calculated adsorption isotherm, kinetics, and thermodynamic parameters show good agreement with the characterization results of adsorbate post-adsorption. The adsorption isotherm is being in congruence with the Langmuir model, and the highest adsorption capacity recorded was 1442.0 mg g−1 at 323 K and pH of 11.0. Adsorption kinetics was better fitted to the pseudo 1st order model than the pseudo 2nd order and Elovich models, which further support the dye physisorption behavior. The initial adsorption rate was influenced by the rapid surface adsorption followed by intraparticle diffusion. The thermodynamic parameters show the spontaneity of the adsorption, and the adsorption proceeds endothermically. The cost analysis shows the economic feasibility of BHB sorbent production for adsorption applications.
中文翻译:

来自海藻酸盐中戊二醛交联植物化学物质的生物复合水凝胶珠可有效去除亚甲蓝
从紫甘蓝中提取的植物化学物质,即类黄酮、酚类和花青素,与海藻酸盐交联,制备生物复合水凝胶珠 (BHB)。BHB 的制备涉及三个连续的步骤:(1) 从紫甘蓝中提取和溶剂还原植物化学物质,(2) 使用戊二醛将植物化学物质交联到海藻酸盐基质中,以及 (3) 在 CaCl2 溶液中形成水凝胶珠。使用扫描电子显微镜 (SEM)、傅里叶变换红外光谱 (FTIR) 和 X 射线衍射 (XRD) 分析对所得 BHB 吸附剂进行了表征。从 SEM 图像中证实了 BHB 的横截面结构。FTIR 峰的改变意味着植物化学化合物成功交联到藻酸盐中。以碱性蓝 9 (BB9) 为模型吸附物进行 BHB 的吸附平衡和动力学研究。BHB 后吸附的 FTIR 表征揭示了参与染料吸附的吸附剂的官能团。计算的吸附等温线、动力学和热力学参数与吸附后吸附的表征结果吻合较好。吸附等温线与 Langmuir 模型一致,在 323 K 和 pH 值为 11.0 时记录的最高吸附容量为 1442.0 mg g-1。与伪 2 级和 Elovich 模型相比,吸附动力学更适合于伪 1 级模型,这进一步支持染料物理吸附行为。初始吸附速率受快速表面吸附和颗粒内扩散的影响。热力学参数显示吸附的自发性,吸附以吸热方式进行。 成本分析显示了 BHB 吸附剂生产用于吸附应用的经济可行性。
更新日期:2021-02-05
中文翻译:

来自海藻酸盐中戊二醛交联植物化学物质的生物复合水凝胶珠可有效去除亚甲蓝
从紫甘蓝中提取的植物化学物质,即类黄酮、酚类和花青素,与海藻酸盐交联,制备生物复合水凝胶珠 (BHB)。BHB 的制备涉及三个连续的步骤:(1) 从紫甘蓝中提取和溶剂还原植物化学物质,(2) 使用戊二醛将植物化学物质交联到海藻酸盐基质中,以及 (3) 在 CaCl2 溶液中形成水凝胶珠。使用扫描电子显微镜 (SEM)、傅里叶变换红外光谱 (FTIR) 和 X 射线衍射 (XRD) 分析对所得 BHB 吸附剂进行了表征。从 SEM 图像中证实了 BHB 的横截面结构。FTIR 峰的改变意味着植物化学化合物成功交联到藻酸盐中。以碱性蓝 9 (BB9) 为模型吸附物进行 BHB 的吸附平衡和动力学研究。BHB 后吸附的 FTIR 表征揭示了参与染料吸附的吸附剂的官能团。计算的吸附等温线、动力学和热力学参数与吸附后吸附的表征结果吻合较好。吸附等温线与 Langmuir 模型一致,在 323 K 和 pH 值为 11.0 时记录的最高吸附容量为 1442.0 mg g-1。与伪 2 级和 Elovich 模型相比,吸附动力学更适合于伪 1 级模型,这进一步支持染料物理吸附行为。初始吸附速率受快速表面吸附和颗粒内扩散的影响。热力学参数显示吸附的自发性,吸附以吸热方式进行。 成本分析显示了 BHB 吸附剂生产用于吸附应用的经济可行性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号